63610-09-3 Usage
Description
Lodoxamidetromethamine, also known as Alomide, is a white crystalline, water-soluble powder that is used in the treatment of various ocular disorders. It is a mast cell stabilizer that inhibits degranulation and the release of histamine and other vasoactive products, similar to sodium cromoglycate. Lodoxamidetromethamine is available as a 0.1% solution and is indicated for the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
Uses
Used in Ophthalmology:
Lodoxamidetromethamine is used as a treatment for ocular disorders such as vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis. It acts as a mast cell stabilizer, inhibiting the release of histamine and other vasoactive products, which helps to alleviate the symptoms of these conditions.
Used in Allergic Conjunctivitis Treatment:
Lodoxamidetromethamine is used as a treatment for allergic conjunctivitis, where it stabilizes mast cells and prevents the release of histamine and other inflammatory mediators, reducing the symptoms of the condition.
Used in Cataract Treatment:
Lodoxamidetromethamine is also used in the treatment of cataracts, although the specific application reason is not provided in the materials.
Used in Antiallergic/Antiasthmatic Research:
Lodoxamidetromethamine is under investigation as an orally active antiallergic/antiasthmatic agent, suggesting potential uses in the treatment of allergic and asthmatic conditions in the future.
Brand names for Lodoxamidetromethamine include Alomide (Alcon) and Almide.
Originator
Upjohn (U.S.A.)
Check Digit Verification of cas no
The CAS Registry Mumber 63610-09-3 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 6,3,6,1 and 0 respectively; the second part has 2 digits, 0 and 9 respectively.
Calculate Digit Verification of CAS Registry Number 63610-09:
(7*6)+(6*3)+(5*6)+(4*1)+(3*0)+(2*0)+(1*9)=103
103 % 10 = 3
So 63610-09-3 is a valid CAS Registry Number.
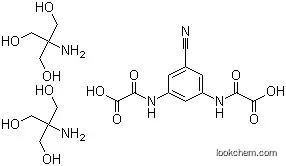
InChI:InChI=1/C11H6ClN3O6.C4H11NO3/c12-7-5(14-8(16)10(18)19)1-4(3-13)2-6(7)15-9(17)11(20)21;5-4(1-6,2-7)3-8/h1-2H,(H,14,16)(H,15,17)(H,18,19)(H,20,21);6-8H,1-3,5H2
63610-09-3Relevant articles and documents
Preparation method of lodoxamide tromethamine intermediate
-
Paragraph 0048; 0049, (2018/12/13)
The invention belongs to the technical field of medicine chemical engineering, and particularly discloses a preparation method of a lodoxamide tromethamine intermediate of lodoxamide. The method comprises the following steps of (1) dissolving 3,5-diamino-4-chlorobenzonitrile into an organic solvent to form a solution A; (2) dissolving oxalyl chloride into the organic solvent to form a solution B;(3) slowly dripping the solution A into the solution B; in the dripping process, controlling the temperature of the reaction liquid at -20 to 0 DEG C; after the reaction is completed, slowly drippinga certain amount of ice water into the reaction liquid; after the dripping is completed, continuously performing stirring for 0.5 to 1h; then, performing suction filtering; drying obtained filter caketo obtain lodoxamide. The reaction steps of the method are few; the operation is facilitated; the yield is greatly improved and can reach 90 percent or higher; meanwhile, the related impurities of the product lodoxamide are few; the product purity is high; the HPLC purity can reach 99.5 percent or higher; great significance is realized on the synthesis of the lodoxamide tromethamine medicine.