-
Name
Acetoxyacetyl chloride
- EINECS 237-542-4
- CAS No. 13831-31-7
- Article Data23
- CAS DataBase
- Density 1.281 g/cm3
- Solubility Reacts with water violently.
- Melting Point 159-160 °C
- Formula C4H5ClO3
- Boiling Point 163.7 °C at 760 mmHg
- Molecular Weight 136.535
- Flash Point 62.5 °C
- Transport Information UN 3265 8/PG 2
- Appearance Clear colorless to pale yellow liquid
- Safety 23-26-27-36/37/39-45-8
- Risk Codes 14-34-36/37
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Acetylchloride, (acetyloxy)- (9CI);Glycoloyl chloride, acetate (6CI,8CI);(Acetyloxy)acetyl chloride;2-Acetoxyacetyl chloride;2-Chloro-2-oxoethylacetate;Acetic acid (chlorocarbonyl)methyl ester;Acetylchloride, 2-(acetyloxy)-;a-Acetoxyacetyl chloride;
- PSA 43.37000
- LogP 0.31490
Synthetic route
-
-
13831-30-6
acetoxyacetic acid

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; acetyl chloride for 1.3h; Heating; | 77.5% |
| With phosphorus trichloride | |
| With thionyl chloride; acetyl chloride |

| Conditions | Yield |
|---|---|
| With pyridine Behandeln des vom Acetylchlorid befreiten Reaktionsgemisches mit Thionylchlorid und wenig Pyridin; | |
| (i), (ii) PCl3; Multistep reaction; | |
| (i), (ii) SOCl2; Multistep reaction; | |
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| (i) H2SO4, (ii) SOCl2; Multistep reaction; |
-
-
180048-73-1
2-hydroxy-2-methyl-1-(4-(methylsulfonyl)-phenyl)propan-1-one

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 20℃; Solvent; | 100% |
| With dmap; triethylamine In dichloromethane at 0℃; | 99.5% |
| With pyridine In acetonitrile | |
| With triethylamine In dichloromethane at 20℃; for 3h; Solvent; Reagent/catalyst; | 207 g |
| With triethylamine In dichloromethane at 20℃; for 4h; Solvent; Reagent/catalyst; | 220 g |
-
-
615-36-1
2-bromoaniline

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
901335-15-7
acetic acid (2-bromo-phenylcarbamoyl)-methyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 2h; | 100% |
| With pyridine In dichloromethane at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; | 100% |
-
-
367-24-8
4-bromo-2-fluoroaniline

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 0.5h; Inert atmosphere; | 100% |
| In chloroform at 20℃; Inert atmosphere; | 100% |
| With potassium carbonate In chloroform | 75% |
| In chloroform at 20℃; for 0.5h; Inert atmosphere; |
-
-
653600-07-8
(cis)-3-(4-fluoro-phenoxy)-8-aza-bicyclo[3.2.1]octane

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
-
-
862682-63-1
2-[4-(piperazin-1-yl)anilino]-4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)pyrimidine

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 18 - 25℃; for 1.25h; | 100% |
-
-
417727-43-6
3-(4-fluoro-benzyl)-3,8-diaza-bicyclo[3.2.1]octane

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
-
-
1065608-29-8
methyl 2-(6-(3-(8-azabicyclo[3.2.1]octan-3-yl)-1,2,4-thiadiazol-5-ylamino)-5-(2-methylpyridin-3-yloxy)pyridin-3-ylthio)acetate

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1065608-34-5
2-(3-(5-(3-(2-methylpyridin-3-yloxy)-5-(pyridin-2-ylthio)pyridin-2-ylamino)-1,2,4-thiadiazol-3-yl)-8-azabicyclo[3.2.1]octan-8-yl)-2-oxoethyl acetate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.0833333h; | 100% |
-
-
264600-27-3
5(S)-(isoxazol-3-ylaminomethyl)-3-(4-(1,2,5,6-tetrahydropyrid-4-yl)phenyl)oxazolidin-2-one trifluoroacetate

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 5(S)-(isoxazol-3-ylaminomethyl)-3-(4-(1,2,5,6-tetrahydropyrid-4-yl)phenyl)oxazolidin-2-one trifluoroacetate With sodium hydrogencarbonate In water; acetone at 0 - 4℃; Stage #2: Acetoxyacetyl chloride In water; acetone at 0 - 5℃; for 0.333333h; | 100% |
-
-
13831-31-7
Acetoxyacetyl chloride

-
-
337519-98-9
3-((E)-3-{4-[1-(t-butoxycarbonyl)piperidin-4-yloxy]phenylamino}-1-propenyl)benzonitrile

-
-
475504-77-9
C30H35N3O6

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine | 100% |
-
-
366804-69-5
(((CH3)3C)C6H2CH2NHCH2CH2NHCH2CH2NHCH2)2(SCH2CH2S)

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1185199-09-0
C58H80N6O18S2

| Conditions | Yield |
|---|---|
| With triethylamine | 100% |

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 3-[1-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine hydrochloride With triethylamine In dichloromethane at 20℃; for 0.5h; Stage #2: Acetoxyacetyl chloride In dichloromethane at 0 - 20℃; for 1h; | 100% |
| Stage #1: 3-[1-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine hydrochloride With triethylamine In dichloromethane at 20℃; for 0.5h; Stage #2: Acetoxyacetyl chloride In dichloromethane at 20℃; for 1h; | 100% |
-
-
1020936-75-7
(2,4-dimethoxybenzyl)-(3-methoxyphenyl)amine

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1318074-43-9
2-acetoxy-N-(2,4-dimethoxybenzyl)-N-(3-methoxyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 4h; Inert atmosphere; | 100% |
-
-
1402049-10-8
4,4-bis-(3,5-di-tert-butyl-4-hydroxyphenylsulfanyl)piperidine-1-carboxylic acid ethyl ester

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1402049-43-7
acetic acid 2-[4,4-bis-(3,5-di-tert-butyl-4-hydroxy-phenylsulfanyl)piperidin-1-yl]-2-oxo-ethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 3h; | 100% |

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
-
-
540-37-4
p-aminoiodobenzene

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1.75h; | 100% |

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; | 100% |
-
-
131613-92-8
[1-((R)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-meth-(E)-ylidene]-(4-methoxy-phenyl)-amine

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In chlorobenzene Microwave irradiation; | 100% |
| With 4-methyl-morpholine Microwave irradiation; stereoselective reaction; | 85% |
-
-
1126795-15-0
N'-((1R,1'R,3r,3'R,5S,5'S)-[3,9'-bi(9'-azabicyclo[3.3.1]nonan)]-3'-yl)benzene-1,2-diamine

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1616764-56-7
2-((2-((1R,1'R,3r,3'R,5S,5'S)-[3,9'-bi(9'-azabicyclo[3.3.1]nonan)]-3'-ylamino)phenyl)amino)-2-oxoethyl acetate

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Cooling with ice; | 99.5% |
-
-
202279-91-2
4-nitro-3-piperidin-1-yl-phenylamine

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
885705-21-5
C15H19N3O5

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 1h; | 99% |
-
-
37441-29-5
5-amino-2,4,6-triiodoisophthalic acid dichloride

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
78314-12-2
2-((3,5-bis (chlorocarbonyl)-2,4,6-triiodophenyl)amino)-2-oxoethyl acetate

| Conditions | Yield |
|---|---|
| In water | 99% |
| In N,N-dimethyl acetamide at 20℃; for 15h; Cooling; | 92% |
| In tetrahydrofuran; n-heptane at 20℃; Product distribution / selectivity; Heating / reflux; | 35% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 0℃; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 0℃; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; diethyl ether at 0 - 20℃; | 99% |
-
-
76801-94-0
5-amino-N,N'-bis[2,3-bis(acetyloxy)propyl]2,4,6-triiodo-1,3-benzenedicarboxamide

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 40℃; for 4h; | 99% |

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
358973-75-8
(-)-(3R,4S)-3-acetoxy-4-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]-1-(3-butenyl)-2-azetidinone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 16h; Staudinger reaction; | 98% |
-
-
264600-99-9
5(R)(N-isoxazol-3-yl-N-(tertbutoxycarbonyl)aminomethyl)-3-(3,5-difluoro-4-(1,2,5,6-tetrahydropyrid-4-yl)phenyl)oxazolidin-2-one hydrochloride

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetone at 0 - 20℃; for 2h; | 98% |
-
-
1253282-56-2
N-(4-hydroxybutyl)-O-benzyl-hydroxylamine

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1253282-57-3
C15H21NO5

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; diethyl ether at 0 - 20℃; | 98% |
-
-
1444335-07-2
5-(benzyloxy)-3,4,6-trimethylpyridine-2-amine

-
-
13831-31-7
Acetoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 98% |
Acetoxyacetyl chloride Chemical Properties
Structure of Acetoxyacetyl chloride (CAS NO.13831-31-7):
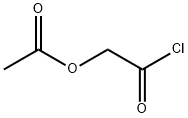
IUPAC Name: (2-chloro-2-oxoethyl) acetate
Empirical Formula: C4H5ClO3
Molecular Weight: 136.5337
EINECS: 237-542-4
Index of Refraction: 1.424
Molar Refractivity: 27.23 cm3
Molar Volume: 106.5 cm3
Polarizability: 10.79×10-24cm3
Surface Tension: 35.2 dyne/cm
Density: 1.281 g/cm3
Flash Point: 62.5 °C
Enthalpy of Vaporization: 40.03 kJ/mol
Boiling Point: 163.7 °C at 760 mmHg
Vapour Pressure: 2.04 mmHg at 25°C
Physical Appearance: Clear colorless to pale yellow liquid
Product Categories: Biochemistry;Reagents for Oligosaccharide Synthesis;Acid Halides;Carbonyl Compounds;Organic Building Blocks
Synonyms of Acetoxyacetyl chloride (CAS NO.13831-31-7): 2-Chloro-2-oxoethyl acetate ; Acetyl chloride, 2-(acetyloxy) ; 2-Acetoxyacetyl chloride .
Canonical SMILES: CC(=O)OCC(=O)Cl
InChI: InChI=1S/C4H5ClO3/c1-3(6)8-2-4(5)7/h2H2,1H3
InChIKey: HZDNNJABYXNPPV-UHFFFAOYSA-N
Acetoxyacetyl chloride Safety Profile
Hazard Codes:  C
C
Risk Statements: 14-34-36/37
R14 :Reacts violently with water.
R34:Causes burns.
R36/37:Irritating to eyes and respiratory system.
Safety Statements: 23-26-27-36/37/39-45-8
S23:Do not breathe vapour.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S27:Take off immediately all contaminated clothing.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S8:Keep container dry.
RIDADR: UN 3265 8/PG 2
WGK Germany: 3
Hazard Note: Corrosive
HazardClass: 8
PackingGroup: II
Related Products
- Acetoxyacetyl chloride
- 138314-11-1
- 138314-12-2
- 13831-59-9
- 13832-70-7
- 138-32-9
- 138330-18-4
- 138331-02-9
- 138343-77-8
- 138343-94-9
- 138344-18-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View