-
Name
Acetyl ketene
- EINECS 211-617-1
- CAS No. 674-82-8
- Article Data28
- CAS DataBase
- Density 1.10 g/cm3
- Solubility soluble in water and organic solvents
- Melting Point -7.5 °C
- Formula C4H4O2
- Boiling Point 127.4 °C at 760 mmHg
- Molecular Weight 84.0746
- Flash Point 97.9 °C
- Transport Information UN 2929 6.1/PG 2
- Appearance colorless liquid
- Safety 3-36/37-33-24/25-23-16
- Risk Codes 10-20-40
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 3-Butenoicacid, 3-hydroxy-, b-lactone (6CI,7CI);4-Methylene-2-oxetanone;Diketene;Ethenone, dimer;Ketene dimer;NSC 93783;
- PSA 26.30000
- LogP 0.44700
Synthetic route

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
13007-90-4
bis(triphenylphosphine)nickel(0) dicarbonyl

-
C
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| With carbon monoxide In toluene High Pressure; 5 atm CO, 50°C, 1 h; pptn. of complex; | A 8% B 83% C n/a |


| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine In hexane at -15 - -10℃; for 3.5h; | 75% |

| Conditions | Yield |
|---|---|
| at 325℃; |

| Conditions | Yield |
|---|---|
| at 110℃; under 15 Torr; |
-
-
463-51-4
Ketene

-
-
106-99-0
buta-1,3-diene

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
66166-61-8
3-vinylcyclobutanone

| Conditions | Yield |
|---|---|
| With nitrogen at 100℃; |


| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| Pd(PCy3)2(dba) In benzene-d6 at 25℃; under 22501.8 Torr; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
38418-24-5
N-(4-bromophenyl)-3-oxobutyramide

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
106-40-1
4-bromo-aniline

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
5437-98-9
4'-methoxyacetoacetanilide

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
25233-46-9
3-oxo-N-m-tolylbutanamide

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
108-44-1
1-amino-3-methylbenzene

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
25233-47-0
N-(3'-methoxyphenyl)-3-oxobutanamide

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
536-90-3
m-Anisidine

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
61579-06-4
N-(3-bromo-phenyl)-3-oxo-butyramide

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
591-19-5
3-bromoaniline

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Equilibrium constant; |
-
-
463-49-0
1,2-propanediene

-
-
106-51-4
p-benzoquinone

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
52727-24-9
Spiro<3.5>nona-5,8-dien-2,7-dion

-
C
-
115077-69-5
3-Methylene-1-oxa-spiro[3.5]nona-5,8-dien-7-one

-
D
-
115077-67-3
C15H12O4

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane at -25℃; under 760 Torr; for 1h; Irradiation; Laser VIS-irradiation, 455-515 nm; | A n/a B n/a C 146 mg D 6.2 mg |

-
-
463-51-4
Ketene

-
-
75-07-0
acetaldehyde

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
C
-
625-33-2
3-penten-2-one

-
-
463-51-4
Ketene

-
-
75-07-0
acetaldehyde

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
C
-
625-33-2
3-penten-2-one

-
-
463-51-4
Ketene

-
-
7664-93-9
sulfuric acid

-
-
67-64-1
acetone

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
108-22-5
Isopropenyl acetate

-
C
-
108-24-7
acetic anhydride

| Conditions | Yield |
|---|---|
| at 55℃; |

-
-
463-51-4
Ketene

-
-
67-64-1
acetone

-
A
-
674-82-8
4-methyleneoxetan-2-one

-
B
-
108-22-5
Isopropenyl acetate

-
C
-
108-24-7
acetic anhydride

| Conditions | Yield |
|---|---|
| at 100℃; |

-
-
112-16-3
n-dodecanoyl chloride

-
-
121-44-8
triethylamine

-
-
75-36-5
acetyl chloride

-
A
-
674-82-8
4-methyleneoxetan-2-one


| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; | 100% |
| In dichloromethane at 0 - 20℃; | 100% |
| In dichloromethane at 0 - 20℃; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
6131-49-3
acetoacetic acid 2-dimethylamino-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 65℃; for 0.5h; | 100% |
| at 70 - 71℃; for 5h; | 99% |
| With triethylamine at 95℃; for 3h; | 84% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
4360-51-4
cinnamylamine

-
-
26226-10-8
3-oxo-N-(3-phenyl-2-propen-1-yl) butylamide

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 70℃; for 3h; | 100% |
| In diethyl ether |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; Inert atmosphere; | 100% |
| In tetrahydrofuran at 0℃; for 0.0833333h; Inert atmosphere; | 100% |
| With triethylamine In methanol; toluene at 0℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium acetate In tetrahydrofuran for 2h; Reflux; | 100% |
| With triethylamine In chloroform for 10h; Ambient temperature; | 96.7% |
| With triethylamine | |
| With dmap |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
| for 2h; Yield given; | |
| In toluene at 100℃; for 1h; | |
| In tert-butyl methyl ether at -5 - 0℃; for 1h; | 49.55 g |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
2033-24-1
cycl-isopropylidene malonate

-
-
84257-12-5
2,2-dimethyl-5-(1-hydroxy-3-oxobutylidene)-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
| Stage #1: 4-methyleneoxetan-2-one; cycl-isopropylidene malonate With triethylamine In dichloromethane at 0 - 20℃; for 2h; Stage #2: With hydrogenchloride In dichloromethane; water | 100% |
| With triethylamine In dichloromethane at 20℃; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
74-93-1
methylthiol

-
-
108615-97-0
4-Methylsulfanylmethyl-oxetan-2-one

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In diethyl ether for 2h; Ambient temperature; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 0.5h; | 100% |
| With sulfuric acid In diethyl ether for 2h; Ambient temperature; | 72% |
| In diethyl ether at 0 - 5℃; |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
111-88-6
Octanethiol

-
-
108615-99-2
4-Octylsulfanylmethyl-oxetan-2-one

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In Petroleum ether at 25℃; for 1h; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
2516-33-8
Cyclopropylmethanol

-
-
86780-84-9
cyclopropylmethyl 3-oxobutanoate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 10h; Ambient temperature; | 100% |
| With sodium hydride at 70 - 80℃; for 2h; | 93% |
| With sodium acetate for 16h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 100% |
| With sodium acetate In acetonitrile for 2h; Heating; | 96% |
| With triethylamine In acetone for 2h; Heating; | 92% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
4503-58-6
benzyl 2-(2-ethoxy-2-ethoxyethyl)-1-hydrazinecarboxylate

-
-
98381-06-7
benzyl 2-acetoacetyl-2-(ethoxycarbonylmethyl)hydrazine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 16h; Heating; | 100% |
| In benzene Yield given; |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
4490-81-7
isopropoxyamine hydrochloride

-
-
133146-80-2
N-Isopropoxy-3-oxo-butyramide

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; toluene at 0 - 5℃; for 0.5h; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
65253-04-5
(-)-8-phenylmenthol

-
-
90358-31-9
(+)-(1R,2S,5R)-8-phenylmenthyl 3-oxobutanoate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 100% |
| With triethylamine In acetone for 3h; Heating; | 95% |
| With sodium acetate In chloroform for 1h; Heating; | 85.9% |
| With sodium acetate In tetrahydrofuran for 2h; Heating; |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
116308-40-8
2-<4-(4-benzhydryl-1-piperazinyl)phenyl>ethyl alcohol

-
-
116308-41-9
2-<4-(4-benzhydryl-1-piperazinyl)phenyl>ethyl acetoacetate

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran 1) ice-salt cooling, 30 min, 2) r.t.; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether Reflux; | 100% |
| In diethyl ether for 3h; Heating / reflux; | 93% |
| In methanol at 0℃; Inert atmosphere; | 46% |
| In methanol for 3h; Ambient temperature; | |
| In methanol at 0℃; Inert atmosphere; |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
81790-27-4
methyl 3-<(benzyloxy)amino>-2-propionate

-
-
81790-28-5
3-[Benzyloxy-(3-oxo-butyryl)-amino]-2-(4-benzyloxy-phenyl)-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran 1.) -78 deg C, 30 min, 2.) 0 deg C, 30 min; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
70491-75-7
(S)-methyl 5,5-dimethyl-1,3-thiazolidine-4-carboxylate

-
-
153823-10-0
methyl (4S)-5,5-dimethyl-3-(3-oxobutanoyl)thiazolidine-4-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 15h; Heating; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
775-15-5
1-benzyl-3-pyrrolidinol

-
-
71863-54-2
1-benzyl-3-acetoacetyloxypyrrolidine

| Conditions | Yield |
|---|---|
| at 70 - 80℃; for 1h; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
14813-01-5
1-benzyl-3-hydroxypiperidine

-
-
85387-34-4
1-benzylpiperidin-3-yl acetoacetate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 10h; Ambient temperature; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 9h; Ambient temperature; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
38552-68-0
3,5-dimethyl-hexa-1,4-dien-3-ol

-
-
664343-03-7
3-oxo-butyric acid 1,3-dimethyl-1-vinyl-but-2-enyl ester

| Conditions | Yield |
|---|---|
| With dmap In diethyl ether at 23℃; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 2h; Heating; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
100-36-7
N,N-diethylethylenediamine

-
-
590424-03-6
N-(2-(diethylamino)ethyl)-3-oxobutanamide

| Conditions | Yield |
|---|---|
| In various solvent(s) | 100% |
| In dichloromethane at 0 - 20℃; for 0.5h; | 100% |
| In tert-butyl methyl ether at 0 - 20℃; | 93% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
4335-60-8, 4360-51-4, 4226-59-9
(E)-cinnamylamine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 70℃; for 3h; | 100% |
| With triethylamine In toluene |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
89639-64-5, 3216-44-2
(E)-3-(thien-2-yl)prop-2-en-1-ol

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 70℃; for 1.5h; | 100% |
| With dmap In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; | 80% |
-
-
674-82-8
4-methyleneoxetan-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 70℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2.5h; Inert atmosphere; | 100% |
-
-
674-82-8
4-methyleneoxetan-2-one

-
-
1517-69-7
(R)-1-phenylethanol

-
-
123261-65-4
(R)-1-phenylethyl acetoacetate

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 20℃; | 100% |
Acetyl ketene Consensus Reports
Acetyl ketene Standards and Recommendations
Acetyl ketene Specification
The Ketene dimer with CAS registry number of 674-82-8 is also known as 2-Oxetanone, 4-methylene-. The IUPAC name is 4-Methylideneoxetan-2-one. It belongs to product categories of Pharmaceutical Intermediates; Aromatic amine products. Its EINECS registry number is 211-617-1. In addition, the formula is C4H4O2 and the molecular weight is 84.07. This chemical is a colorless liquid and should be stored in sealed containers in cool, dry place and away from oxidizing agents, acids, bases and amines.
Physical properties about Ketene dimer are: (1)ACD/LogP: 0.27; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.27; (4)ACD/LogD (pH 7.4): 0.27; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 33.28; (8)ACD/KOC (pH 7.4): 33.28; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Index of Refraction: 1.449; (13)Molar Refractivity: 19.91 cm3; (14)Molar Volume: 74.1 cm3; (15)Surface Tension: 28.5 dyne/cm; (16)Density: 1.13 g/cm3; (17)Flash Point: 97.9 °C; (18)Enthalpy of Vaporization: 36.8 kJ/mol; (19)Boiling Point: 127.4 °C at 760 mmHg; (20)Vapour Pressure: 11.1 mmHg at 25 °C.
Preparation of Ketene dimer: it is prepared by thermal decomposition by acetic acid, acetone, acetic anhydride and acetate. Acetic pyrolysises at 750-780 °C in the presence of triethyl phosphate to get ketene, then polymerizes at 8-10 °C to get the product.
CH3COOH→CH2CO→C4H4O2
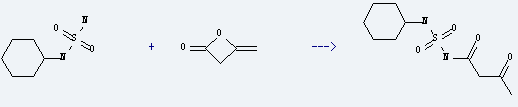
Uses of Ketene dimer: it is used to produce N-acetoacetyl-N'-cyclohexylsulfamide by reaction with cyclohexyl-sulfamide. The reaction occurs with reagent aq.NaOH at ambient temperature for 2 hours. The yield is about 36%. This chemical is also used as raw materials of fine chemicals, dyes, pharmaceuticals, pesticides, food and feed additives, additives and others.
When you are using this chemical, please be cautious about it. As a chemical, it is flammable and harmful by inhalation. There is limited evidence of a carcinogenic effect. During using it, wear suitable protective clothing and gloves. Avoid contact with skin and eyes. What's more, take precautionary measures against static discharges and do not breathe gas/fumes/vapour/spray. After using it, keep it in a cool place and keep away from sources of ignition.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C=C1CC(=O)O1
2. InChI: InChI=1S/C4H4O2/c1-3-2-4(5)6-3/h1-2H2
3. InChIKey: WASQWSOJHCZDFK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC50 | inhalation | 3gm/m3/2H (3000mg/m3) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 301, 1986. | |
| guinea pig | LDLo | skin | 10mL/kg (10mL/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| mouse | LDLo | oral | 800mg/kg (800mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rabbit | LD50 | skin | 2830uL/kg (2.83mL/kg) | Toxicology and Applied Pharmacology. Vol. 28, Pg. 313, 1974. | |
| rat | LCLo | inhalation | 20000ppm/1H (20000ppm) | Industrial Hygiene Foundation of America, Chemical and Toxicological Series, Bulletin. Vol. 6, Pg. 1, 1967. | |
| rat | LD50 | oral | 560uL/kg (0.56mL/kg) | Toxicology and Applied Pharmacology. Vol. 28, Pg. 313, 1974. |
Related Products
- Acetyl bromide
- Acetyl bromide, 2-bromo-2,2-difluoro-
- Acetyl cedrene
- Acetyl cedrene
- Acetyl chloride
- Acetyl chloride,2-(2,4-dichlorophenoxy)-
- Acetyl chloride,2-(4-chlorophenoxy)-
- Acetyl chloride,2-(4-pyridinylthio)-
- Acetyl chloride,2-(chlorosulfonyl)-
- Acetyl chloride,2-bromo-2,2-difluoro-
- 67482-93-3
- 67483-13-0
- 67485-29-4
- 67488-50-0
- 6748-91-0
- 67491-43-4
- 67492-50-6
- 674-97-5
- 67498-53-7
- 6750-04-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View