-
Name
Acetylene
- EINECS 200-816-9
- CAS No. 74-86-2
- Article Data1178
- CAS DataBase
- Density 0.568 g/cm3
- Solubility 0.106 g/100 mL in water
- Melting Point -88°C
- Formula C2H2
- Boiling Point -28°C
- Molecular Weight 26.0379
- Flash Point -18°C
- Transport Information UN 1001
- Appearance colorless gas with a faint garlic-like odor
- Safety 9-16-33
- Risk Codes 5-6-12
-
Molecular Structure
-
Hazard Symbols
 F+
F+
- Synonyms Acetylene(8CI);Vinylene (7CI);Ethine;Narcylen;
- PSA 0.00000
- LogP 0.24940
Synthetic route
-
-
188290-36-0
thiophene

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| under 1 Torr; Product distribution; plasma desulfurization: 10-100 W; | 100% |

| Conditions | Yield |
|---|---|
| With water Hydrolysis; | 100% |
| With water In water decompn.;; | |
| With hydrogen In neat (no solvent) byproducts: Ca; at 2275°C pressure of H2 1 atm;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.1% B 1.13% C 2.2% D 96.5% |
| In neat (no solvent) hydrolysis of the annealed sample in water vapor (11.0E2 Nm**2); | A 0.11% B 5.2% C 2.5% D 92.2% |

-
-
1165952-92-0
cyclohexa-1,4-diene

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With hydrogen; silica gel; Pt/Al2O3 at 170℃; for 48h; examination of hydrogenation var. time and reag;; | 95% |

| Conditions | Yield |
|---|---|
| potassium hydroxide In water at 80 - 84℃; for 12 - 45h; Product distribution / selectivity; | A 93.8% B 95% |
| With [Mg0751Al0.249(OH)2](C(01)O3)25*0.71H2O at 149.84℃; under 3000.3 Torr; Kinetics; Reagent/catalyst; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.03% B 2.7% C 2.7% D 94.6% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.12% B 4.6% C 2.8% D 92.4% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**-2); | A 0.9% B 1.8% C 5.21% D 92.1% |


| Conditions | Yield |
|---|---|
| In gas Quantum yield; Mechanism; Irradiation; | A n/a B 90% C n/a |


| Conditions | Yield |
|---|---|
| potassium fluoride on basic alumina at 100℃; for 2h; Product distribution / selectivity; Neat (no solvent); | A 90% B n/a |
| With potassium hydroxide at 100℃; for 2h; Neat (no solvent); | A 89% B n/a |

-
-
7732-18-5
water

-
A
-
34557-54-5
methane

-
B
-
74-84-0
ethane

-
C
-
74-98-6
propane

-
D
-
74-85-1
ethene

-
E
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.24% B 6.77% C 0.14% D 2.54% E 89.5% |

-
-
1197-51-9
N-propargylbenzylamine

-
-
693-02-7
hex-1-yne

-
A
-
1379038-80-8
N-benzylhept-2-yn-1-amine

-
B
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With Lu(N(SiMe3)2)3 In toluene at 130℃; for 12h; Inert atmosphere; Glovebox; Schlenk technique; Sealed tube; | A 87% B n/a |

-
-
75-75-2
methanesulfonic acid

-
-
603-35-0
triphenylphosphine

-
-
1066-54-2
trimethylsilylacetylene

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 2h; Addition; Heating; | 86% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.11% B 7.27% C 7.85% D 85% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran | A 84% B n/a |

-
-
540-49-8
cis+trans-dibromoethylene

-
-
65567-06-8, 4541-02-0
lithium diphenylphosphide

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Product distribution; Heating; reagent for metal-halogen exchange (reactions with other aliphatic dihalides); | 80% |
-
-
540-49-8
cis+trans-dibromoethylene

-
A
-
40612-18-8
(E)-1,2-bis(diphenylphosphoryl)ethene

-
B
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With lithium diphenylarsenide In tetrahydrofuran for 1h; Heating; | A n/a B 80% |
-
-
79-01-6
Trichloroethylene

-
A
-
156-59-2
cis-1,2-Dichloroethylene

-
B
-
75-01-4
chloroethylene

-
C
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With iron sulfide In water for 2922h; pH=8.3; Kinetics; Product distribution; Dehydrochlorination; | A 6% B 1% C 65% |

-
-
34557-54-5
methane

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With Ba0.5Sr0.5Co0.78W0.02Fe0.2O3 at 1200℃; | 60% |
| With chlorine durch Vercracken; | |
| im Wechselstrombogen (620-2100 V) bei 68-760 Torr und verschiedener Durchflussgeschwindigkeit, auch bei Verduennung mit Wasserstoff; |
-
-
123625-05-8
3-Azido-4-chloro-buta-1,2-diene

-
A
-
124318-33-8
3-Chloromethyl-2-methylene-2H-azirine

-
B
-
107-14-2
chloroacetonitrile

-
C
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| Irradiation; | A 60% B n/a C n/a |


| Conditions | Yield |
|---|---|
| at 450℃; under 0.0001 Torr; for 4h; Title compound not separated from byproducts; | A n/a B 60% C n/a |

-
-
123625-04-7
3-Azido-4-methoxy-buta-1,2-diene

-
A
-
1738-36-9
2-methoxyacetonitrile

-
B
-
124318-32-7
3-Methoxymethyl-2-methylene-2H-azirine

-
C
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| Irradiation; | A n/a B 59% C n/a |

-
-
88596-79-6
azidopropadiene

-
A
-
124318-35-0
3-Methyl-2H-azirine-2-carbonitrile

-
B
-
107-13-1
acrylonitrile

-
C
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| at -70℃; Irradiation; | A 57% B 30% C n/a |

-
-
127-18-4
1,1,2,2-tetrachloroethylene

-
A
-
156-59-2
cis-1,2-Dichloroethylene

-
B
-
79-01-6
Trichloroethylene

-
C
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With iron sulfide In water for 2922h; pH=8.3; Kinetics; Product distribution; Dehydrochlorination; | A 1% B 1% C 56% |
| With iron sulfide; rac-cysteine In water for 2922h; pH=8.3; Kinetics; Product distribution; Dehydrochlorination; | A 1% B 2% C 19% |

-
-
616-10-4
bicyclo(4,2,0)oct-7-ene

-
A
-
3806-59-5
1,3-cyclooctadiene

-
B
-
3806-60-8
(1E,3Z)-1,3-cyclooctadiene

-
C
-
74-86-2
acetylene

-
D
-
110-83-8
cyclohexene

| Conditions | Yield |
|---|---|
| In pentane for 0.25h; Ambient temperature; Irradiation; | A 15% B 6.6% C n/a D 52% |
| In pentane for 0.166667h; Product distribution; Quantum yield; Ambient temperature; Irradiation; variation of irradiation time; | A 15% B 6.6% C n/a D 52% |
| In pentane for 0.166667h; Ambient temperature; Irradiation; | A 18% B 6.6% C n/a D 51% |

-
-
75-77-4
chloro-trimethyl-silane

-
-
1066-26-8
sodium acetylide

-
A
-
74-86-2
acetylene

-
B
-
1066-54-2
trimethylsilylacetylene

-
C
-
14630-40-1
Bis(trimethylsilyl)ethyne

| Conditions | Yield |
|---|---|
| In various solvent(s) at 100℃; | A 0.232 mol B 29% C 50% |


| Conditions | Yield |
|---|---|
| With lithium diphenylarsenide In tetrahydrofuran for 1h; Product distribution; Heating; various amounts of Ph2AsLi (2 equiv or 1 equiv), other reagent (lithium diphenylphosphide); | 46% |
| With lithium diphenylphosphide In tetrahydrofuran Heating; | |
| In various solvent(s) Product distribution; Further Variations:; laser light polarization; UV-irradiation; |
-
-
96308-43-9
trans-bicyclo<5.2.0>non-8-ene

-
A
-
628-92-2
Cycloheptene

-
B
-
3726-88-3
(Z,Z)-1,3-cyclononadiene

-
C
-
3776-88-3
cis,trans-cyclonona-1,3-diene

-
D
-
54211-15-3, 115411-11-5
ο-methylenebicyclo<5.1.0>octane

-
E
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In pentane at 23℃; Product distribution; Quantum yield; Mechanism; Irradiation; variation of wave lengths; | A 15% B 15% C 46% D 18% E n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) plasma beams (from vaporized graphite cathode) with H2 as plasma gas yield C2H2 in the quenched gases;; | 46% |
-
-
291-64-5
cycloheptane

-
A
-
50-00-0
formaldehyd

-
B
-
64-18-6
formic acid

-
C
-
54894-22-3
Cycloheptyl Nitrate

-
D
-
74-86-2
acetylene

-
E
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With water; nitrogen(II) oxide for 5.15h; Product distribution; Irradiation; further reaction times, initial conc.,; | A 0.6% B 1.3% C 3.2% D 7.4% E 45% |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 100% |
| With tetra(n-butyl)ammonium hydroxide In water; dimethyl sulfoxide at 5℃; for 1h; Favorskii-Babayan Synthesis; | 90% |
| With diethyl ether; potassium 2-methylbutan-2-olate |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 100% |
| With potassium hydroxide; diethyl ether at 0℃; under 7355.08 Torr; | |
| With diethyl ether; potassium 2-methylbutan-2-olate |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 100% |
| With tetra(n-butyl)ammonium hydroxide In water; dimethyl sulfoxide at 5℃; for 1h; Favorskii-Babayan Synthesis; | 78% |
| With sodium amide |

| Conditions | Yield |
|---|---|
| Stage #1: estra-4-ene-3,17-dione With potassium hydroxide In toluene; tert-butyl alcohol under 760.051 Torr; for 0.166667h; Reflux; Industrial scale; Stage #2: acetylene In tert-butyl methyl ether; toluene for 6h; Industrial scale; | 100% |
| With potassium tert-butylate; acetone |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; oxygen at 199.84℃; under 750.075 Torr; for 12h; Reagent/catalyst; Flow reactor; Inert atmosphere; | 100% |
| With hydrogenchloride at 180℃; Reagent/catalyst; | 99.9% |
| With hydrogenchloride In water at 250℃; under 825.083 - 900.09 Torr; Temperature; Reagent/catalyst; | 68% |
-
-
37935-47-0
2-(5-bromopentyloxy)tetrahydropyran

-
-
74-86-2
acetylene

-
-
37011-86-2
tetrahydro-2-(6-heptynyloxy)-2H-pyran

| Conditions | Yield |
|---|---|
| Stage #1: acetylene With n-butyllithium In tetrahydrofuran; hexane at -10℃; for 0.5h; Stage #2: 2-(5-bromopentyloxy)tetrahydropyran In tetrahydrofuran; hexane; dimethyl sulfoxide at 20℃; | 100% |
| With lithium; dimethyl sulfoxide In ammonia at -33℃; for 2h; | 90% |
| With ammonia; iron(III) chloride; lithium 1.) -35 deg C, 15 min, 2.) THF, DMSO; Yield given. Multistep reaction; | |
| With ammonia; iron(III) chloride; lithium 1.) -35 deg C, 75 min, 2.) THF, DMSO, -35 deg C, 4 h; Yield given. Multistep reaction; |
-
-
110547-61-0
(Z)-1,1-dimesityl-2-neopentylidenesilirane

-
-
74-86-2
acetylene

-
-
122507-63-5
1,1-dimesityl-4-E-neopentylidene-1-silacyclopent-2-ene

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In toluene at 80℃; for 4h; | 100% |
| tetrakis(triphenylphosphine) palladium(0) at 80℃; Yield given; |

| Conditions | Yield |
|---|---|
| at 460℃; | 100% |
| In methanol at 440℃; for 1.28333h; | 89.5% |
| With Diethyl disulfide at 510℃; | |
| With methanol thermolysis; | |
| With methanol Thermolysis; |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 160 - 165℃; under 12160 Torr; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 100% |

| Conditions | Yield |
|---|---|
| With benzo[1,3,2]dioxaborole In tetrahydrofuran Heating; | 100% |

| Conditions | Yield |
|---|---|
| With In(OSO2CF3)3; 3 A molecular sieve; 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 100℃; under 760 Torr; for 30h; | 100% |
| With indium(III) triflate; 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 100℃; Molecular sieve; | 100% |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
124-38-9
carbon dioxide

-
-
74-86-2
acetylene

-
-
1577-31-7
(Z)-hept-2-enoic acid

| Conditions | Yield |
|---|---|
| Stage #1: n-butyllithium; acetylene With copper(l) iodide In diethyl ether; hexane at -50℃; for 0.5h; Stage #2: carbon dioxide In diethyl ether; hexane at -50℃; for 2h; Further stages.; | 100% |


| Conditions | Yield |
|---|---|
| bis(triphenylphosphine)carbonyliridium(I) chloride In neat (no solvent) tube was charged with Ir-complex, evacuated, alkyne and borane were condensed into tube, mixt. was warmed to room temp., heated at 75°C for 4 h; purified by GLC on the TCP column (80°C); elem. anal.; | 100% |
-
-
157072-60-1, 61521-25-3, 166941-05-5, 16971-33-8
(carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II)

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In dichloromethane C2H2-atmosphere; stirring (15 min); crystn. on EtOH addn. and crystn., filtering, washing (EtOH, petroleum ether), drying; elem. anal.; | 100% |
| In dichloromethane Inert atmosphere; | 68% |
| In dichloromethane acetylene was bubbled through a soln. of Ru-complex in CH2Cl2 for 30 min with stirring and mild heating; unidentified mixt. of products of polyinsertion of acetylene was also formed; soln. was chromd. on a Florisil column (eluent CH2Cl2), alkenyl complexwas isolated from the eluate by concn. and pptn. with petroleum ether; elem. anal.; | 55% |
-
-
79235-76-0
(5,10,15,20-tetramesitylporphyrinato)ruthenium(II)

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In benzene Exposure of starting soln. to C2H2 (1 atm) and shaking. Upon leaving under room light for 16 h, the soln. turns from dark brown to greenish-black.; Removal of solvent by evacuation, drying of resulting solid (vac., 24 h, 25°C), elem. anal.; | 100% |
-
-
907590-97-0
1,3-bis-(2,6-diisopropyl-phenyl)-6-methyl-4-methylene-1,2,3,4-tetrahydro-[1,3,2]diazasiline

-
-
74-86-2
acetylene

-
-
1034170-45-0
C5H6(NC6H3(CH(CH3)2)2)2SiC2H2

| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 3h; | 100% |

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 at -60℃; | 100% |
-
-
1012314-44-1
[(η5-C5Me5)Co-(η5-1-phenylpentadienyl)]+ BF4-

-
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 60℃; for 96h; | 100% |

| Conditions | Yield |
|---|---|
| for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| In hexane at 20℃; under 760.051 Torr; for 0.166667h; Inert atmosphere; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| In hexane at 20℃; under 760.051 Torr; for 0.25h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: acetylene With potassium tert-butylate In tetrahydrofuran at 0℃; for 1h; Stage #2: cyclopentanone In tetrahydrofuran at 0 - 20℃; | 99.9% |
| With potassium tert-butylate In tetrahydrofuran at 10 - 15℃; for 0.333333h; | 90% |
| With potassium hydroxide In dimethyl sulfoxide at 15 - 17℃; for 1h; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: acetylene With potassium tert-butylate In tetrahydrofuran at 0℃; for 1h; Stage #2: cyclohexanone In tetrahydrofuran at 0 - 20℃; | 99.3% |
| With potassium hydroxide In dimethyl sulfoxide at 15 - 17℃; for 2h; | 98% |
| Stage #1: acetylene With ammonia; sodium at -78℃; Stage #2: cyclohexanone In diethyl ether at -78℃; for 12h; | 97% |

| Conditions | Yield |
|---|---|
| With sodium In methanol at 0 - 5℃; for 1h; Solvent; Reagent/catalyst; Autoclave; Inert atmosphere; Large scale; | 99.2% |
| Stage #1: acetylene With sodium amide In ammonia at -50℃; for 0.5h; Stage #2: acetone at -50 - 25℃; | 87.9% |
| Stage #1: acetylene With potassium hydroxide monohydrate In dimethyl sulfoxide at 10 - 15℃; for 7h; Stage #2: acetone In dimethyl sulfoxide at 10 - 15℃; for 9h; Temperature; | 82% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 0 - 5℃; for 0.5h; Inert atmosphere; Large scale; | 99.2% |
| Stage #1: acetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: benzophenone In tetrahydrofuran; hexane at -78 - 20℃; | 96% |
| With sodium hydroxide; tetrabutylammomium bromide In toluene for 2h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 0 - 5℃; for 0.5h; Inert atmosphere; | 99.1% |
| 86% | |
| With diethyl ether unter Zusatz von Kaliumhydroxid; |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol at 0 - 5℃; for 0.5h; Inert atmosphere; | 99.1% |
| Addition; | 74% |
| With lithium amide In ammonia | |
| With ammonia; lithium; ferric nitrate In diethyl ether |
Acetylene History
In 1836, Acetylene was discovered by Edmund Davy who identified it as a "new carburet of hydrogen".
In 1860, it was rediscovered by French chemist Marcellin Berthelot, who coined the name "acetylene". Berthelot was able to prepare this gas by passing vapours of organic compounds (methanol, ethanol, etc.) through a red-hot tube and collecting the effluent. He also found acetylene was formed by sparking electricity through mixed cyanogen and hydrogen gases. He was also able to form acetylene directly by combining pure hydrogen with carbon using electrical discharge of a carbon arc.
Acetylene Consensus Reports
Reported in EPA TSCA Inventory.
Acetylene Standards and Recommendations
OSHA PEL: CL 2500 ppm
ACGIH TLV: Simple asphyxiant
NIOSH REL: (Acetylene) 10H TWA no exposure >2500 ppm
DOT Classification: Forbidden; DOT Class 2.1; Label: Flammable Gas
Acetylene Specification
The Acetylene, with the CAS registry number 74-86-2, is also known as Ethyne. It belongs to the product categories of Industrial/Fine Chemicals; Organics. Its EINECS registry number is 200-816-9. This chemical's molecular formula is C2H2 and molecular weight is 26.04. Its IUPAC name is called acetylene. What's more, this chemical's classification code is Human Data. It is a hydrocarbon and the simplest alkyne. It is unstable in pure form and thus is usually handled as a solution.
Physical properties of Acetylene: (1)ACD/LogP: 0.37; (2)ACD/LogD (pH 5.5): 0.37; (3)ACD/LogD (pH 7.4): 0.37; (4)ACD/BCF (pH 5.5): 1.13; (5)ACD/BCF (pH 7.4): 1.13; (6)ACD/KOC (pH 5.5): 37.87; (7)ACD/KOC (pH 7.4): 37.87; (8)Index of Refraction: 1.315; (9)Molar Refractivity: 8.96 cm3; (10)Molar Volume: 45.7 cm3; (11)Surface Tension: 16 dyne/cm; (12)Density: 0.568 g/cm3; (13)Enthalpy of Vaporization: 21.12 kJ/mol; (14)Vapour Pressure: 69700 mmHg at 25°C.
Preparation of Acetylene: Today acetylene is mainly manufactured by the partial combustion of methane or appears as a side product in the ethylene stream from cracking of hydrocarbons. It was prepared by the hydrolysis of calcium carbide. calcium carbide production requires extremely high temperatures, ~2000 °C, necessitating the use of an electric arc furnace:
CaC2 + 2H2O → Ca(OH)2 + C2H2
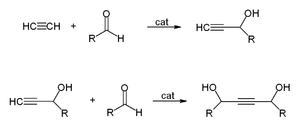
Uses of Acetylene: this colorless gas is widely used as a fuel and a chemical building block. Walter Reppe discovered that in the presence of metal catalysts, acetylene can react to give a wide range of industrially significant chemicals. It can react with alcohols, hydrogen cyanide, hydrogen chloride, or carboxylic acids to give vinyl compounds; with aldehydes to give ethynyl diols; with carbon monoxide to give acrylic acid, or acrylic esters, which can be used to produce acrylic glass; cyclicization to give benzene and cyclooctatetraene. 1,4-Butynediol is produced industrially in this way from formaldehyde and acetylene:
When you are using this chemical, please be cautious about it as the following:
This chemical is extremely flammable. It may explosive with or without contact with air. In addition, you should keep container in a well-ventilated place and away from sources of ignition - No smoking. When using it, please take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C#C
(2)InChI: InChI=1S/C2H2/c1-2/h1-2H
(3)InChIKey: HSFWRNGVRCDJHI-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | LCLo | inhalation | 50pph/5M (500000ppm) | Tabulae Biologicae. Vol. 3, Pg. 231, 1933. | |
| human | TCLo | inhalation | 20pph (200000ppm) | BEHAVIORAL: HEADACHE LUNGS, THORAX, OR RESPIRATION: DYSPNEA | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 67, 1969. |
| mammal (species unspecified) | LCLo | inhalation | 50pph/5M (500000ppm) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 138, Pg. 65, 1928. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View