-
Name
VIOLURIC ACID
- EINECS 201-741-4
- CAS No. 87-39-8
- Article Data16
- CAS DataBase
- Density 2.19 g/cm3
-
Solubility
Slightly soluble
- Hazard Symbols UN NO.
- Synonyms Alloxan,5-oxime (8CI); Violuric acid (6CI,7CI); 5-(Hydroxyimino)barbituric acid;5-Isonitrosobarbituric acid; NSC 56338; VLA
- PSA 107.86000
- LogP -1.15980
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium nitrite In water at 10 - 30℃; for 16h; Reagent/catalyst; | 85.2% |
| With potassium nitrite | |
| With sodium perchlorate; sodium nitrite at 25℃; Kinetics; Mechanism; Concentration; pH-value; Reagent/catalyst; Acidic aq. solution; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; water | |
| With hydroxylamine hydrochloride; water at 60 - 70℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium chlorate man erwaermt die von ueberschuessigem Chlor befreite Loesung mit salzsaurem Hydroxylamin; |
-
-
74646-24-5
alloxan-4-oxime

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With nitric acid | |
| With potassium nitrite; acetic acid | |
| With cis-nitrous acid |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; water |

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With potassium cyanide |

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With glycerol |
-
-
412341-12-9
5-nitroso-6-aminopyrimidine

-
-
87-39-8
violuric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water |

| Conditions | Yield |
|---|---|
| In methanol; water | 90% |

| Conditions | Yield |
|---|---|
| In ethanol | 73% |

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 72% |

| Conditions | Yield |
|---|---|
| In ethanol | 71% |
-
-
87-39-8
violuric acid

-
-
613-13-8
2-aminoanthracene

-
-
196872-60-3
4H-2,4,5,14-Tetraaza-pentaphene-1,3-dione

| Conditions | Yield |
|---|---|
| In acetic acid for 3h; Heating; | 70% |
-
-
87-39-8
violuric acid

-
-
1052111-61-1
ammonium pyrimidine-2,4-5,6(1H,3H)-tetrone 5-oximate

| Conditions | Yield |
|---|---|
| With ammonium carbonate In water | 70% |

| Conditions | Yield |
|---|---|
| In ethanol; water | 70% |

| Conditions | Yield |
|---|---|
| In ethanol | 66% |

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 61% |

| Conditions | Yield |
|---|---|
| In ethanol | 61% |

| Conditions | Yield |
|---|---|
| In ethanol | 61% |

| Conditions | Yield |
|---|---|
| In methanol stoich., ligand and Re complex reacted in CH3OH at 50-60°C under stirring for 3 h; pptd.(hexane), filtered, washed (EtOH, Et2O), dried (air), recrystd.(acetone/H2O 1:1), elem. anal.; | 60% |


| Conditions | Yield |
|---|---|
| With acetic acid for 0.75h; Heating; | 58% |

| Conditions | Yield |
|---|---|
| With acetic acid Reflux; | 58% |

| Conditions | Yield |
|---|---|
| at 155℃; for 0.75h; | 57% |

| Conditions | Yield |
|---|---|
| In ethanol | 55% |

| Conditions | Yield |
|---|---|
| In ethanol | 53% |

| Conditions | Yield |
|---|---|
| With acetic acid Reflux; | 47% |

| Conditions | Yield |
|---|---|
| With acetic acid Reflux; | 46% |
-
-
87-39-8
violuric acid

-
-
1262317-11-2
tert-butyl (2-((3-(cyclopentyloxy)-4-methylphenyl)amino)ethyl)carbamate

-
-
1262317-12-3
tert-butyl (2-(8-(cyclopentyloxy)-7-methyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethyl)carbamate

| Conditions | Yield |
|---|---|
| In ethanol at 145℃; for 1.5h; Microwave irradiation; | 38% |

| Conditions | Yield |
|---|---|
| In water for 3h; | 37% |

-
-
87-39-8
violuric acid

-
-
1416815-95-6
C24H43N

-
-
1416815-96-7
10-hexadecyl-7,8-dimethyl-10H-benzo[g]pteridine-2,4-dione

| Conditions | Yield |
|---|---|
| With acetic acid for 48h; Reflux; Inert atmosphere; Darkness; | 30% |
-
-
87-39-8
violuric acid

-
-
134-32-7
1-amino-naphthalene

-
-
196872-59-0
11H-naphtho[2,1-g]pteridine-8,10-dione

| Conditions | Yield |
|---|---|
| In acetic acid for 24h; Heating; | 11% |

| Conditions | Yield |
|---|---|
| With acetic anhydride |
-
-
1004-38-2
2,4,6-triaminopyrimidine

-
-
87-39-8
violuric acid

-
-
7147-37-7
6,8-diamino-1H-pyrimido[5,4-g]pteridine-2,4-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate; acetic acid |
-
-
873-83-6
4-Amino-2,6-dihydroxypyrimidine

-
-
87-39-8
violuric acid

-
-
5807-13-6
1,3,6,8-tetrahydro-2,4,5,7-pyrimido<5,4-g>pteridinetetrone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid |
Alloxan 5-oxime Chemical Properties
Molecular Formula: C4H3N3O4
Molar mass: 157.08 g/mol
EINECS: 201-741-4
Density: 2.19 g/cm3
Flash Point: 252.6 °C
Index of Refraction: 1.813
Boiling Point: 494.1 °C at 760 mmHg
Vapour Pressure: 7.79E-12 mmHg at 25°C
Melting point: 240-250 °C (dec.)
Appearance: Light yellow crystalline powder
Structure of Alloxan 5-oxime (87-39-8):
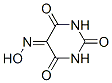
XLogP3-AA: -0.2
H-Bond Donor: 3
H-Bond Acceptor: 5
Systematic Name Alloxan 5-oxime (87-39-8): 5-Hydroxyiminohexahydropyrimidine-2,4,6-trione
SMILES: O=C1C(=N\O)\C(=O)NC(=O)N1
InChI: InChI=1/C4H3N3O4/c8-2-1(7-11)3(9)6-4(10)5-2/h11H,(H2,5,6,8,9,10)
InChIKey: JMUJZTASUDOAGC-UHFFFAOYAY
Std. InChI: InChI=1S/C4H3N3O4/c8-2-1(7-11)3(9)6-4(10)5-2/h11H,(H2,5,6,8,9,10)
Std. InChIKey: JMUJZTASUDOAGC-UHFFFAOYSA-N
Alloxan 5-oxime Toxicity Data With Reference
| 1. | ivn-mus LD50:100 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#05202 . |
Alloxan 5-oxime Consensus Reports
Reported in EPA TSCA Inventory.
Alloxan 5-oxime Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes: Xi![]()
Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
Alloxan 5-oxime Specification
Alloxan 5-oxime (87-39-8) also can be called Violuric acid ; 5-(Hydroxyimino)pyrimidine-2,4,6(1H,3H,5H)-trione ; 2,4,5,6(1H,3H)-pyrimidinetetrone, 5-oxime ; Pyrimidin-2,4,5,6(1H,3H)-tetron-5-oxim and 5-(Hydroxyimino)-2,4,6(1H,3H,5H)-pyrimidinetrione .
Related Products
- Alloxan
- Alloxan 5-oxime
- Alloxan monohydrate
- 87403-73-4
- 874-05-5
- 87407-12-3
- 874-10-2
- 874119-56-9
- 87-41-2
- 87413-09-0
- 874-14-6
- 874-17-9
- 874-18-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View