-
Name
Alloxan
- EINECS 200-062-0
- CAS No. 50-71-5
- Article Data2
- CAS DataBase
- Density 1.681g/cm3
-
Solubility
- Hazard Symbols UN NO.
- Synonyms Alloxan(8CI); 2,4,5,6-Pyrimidinetetrone; 2,4,5,6-Tetraoxohexahydropyrimidine;Alloxane; Mesoxalylcarbamide; Mesoxalylurea; NSC 7169; Pyrimidinetetrone
- PSA 129.26000
- LogP -1.67810
Synthetic route

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide In acetic acid |

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 24h; | 92% |
-
-
95-54-5
1,2-diamino-benzene

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

-
-
490-59-5
alloxazine

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 86% |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boron trioxide; acetic acid at 20℃; for 18h; | 82% |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boron trioxide; acetic acid at 20℃; for 14h; | 78% |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 75% |
-
-
771-97-1
2,3-Diaminonaphthalene

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

-
-
4794-65-4
naphtho[2,3-g]pteridine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 73% |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With water In water gradual addn. of an aq. soln. of CuCl2*2H2O to an aq. soln. of alloxane; simultaneous addn. of 10% aq. NaOH (->pH=7.5); pH=5 or pH=6 for a few hours;; ppt.; washing with cold H2O; drying in vac. at ambient temp. over CaCl2;; | 72% |
| With H2O In water gradual addn. of an aq. soln. of CuCl2*2H2O to an aq. soln. of alloxane; simultaneous addn. of 10% aq. NaOH (->pH=7.5); pH=5 or pH=6 for a few hours;; ppt.; washing with cold H2O; drying in vac. at ambient temp. over CaCl2;; | 72% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water addition of CoCl2 in H2O to a solution of alloxane in H2O under addition of NaOH-solution until pH=7.5; then pH=5-6; seperation of the ppt. after some hours;; washing the product with cold H2O; drying in vac. at normal temperature over CaCl2;; | 69% |
| With NaOH In water |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water gradual addn. of equimolar amt. of aq. Ni(NO3)2*6H2O soln. to aq. soln. of alloxan, addn. of 10% NaOH soln. to pH 7.5, then adjusting to pH 5-6;; pptn., sepg. after several hours, washing with cold H2O, drying in vac. at ambient temp. over CaCl2;; | 66% |
-
-
76179-40-3
2-amino-4,5-difluoroaniline

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 58% |

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 57% |
-
-
38423-40-4
1-(N-octylamino)-2-aminobenzene

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boric acid; acetic acid at 60℃; for 1h; | 41% |
-
-
320406-61-9
N-butyl-4-trifluoromethylbenzene-1,2-diamine

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With boric acid; acetic acid at 70℃; for 3h; | 39% |

| Conditions | Yield |
|---|---|
| With boric acid; acetic acid at 60℃; for 1h; | 33.7% |
-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

-
-
18514-52-8
2,3-diaminomaleonitrile

-
-
55408-53-2
2,4-dioxo-1,2,3,4-tetrahydropteridine-6,7-dicarbonitrile

| Conditions | Yield |
|---|---|
| With boric acid In acetic acid at 60℃; for 15h; Inert atmosphere; | 32% |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| With H2O In water pptn. from alloxane soln. with Hg(NO3)2;; |

-
-
50-71-5
5,5-dihydroxypyrimidine-2,4,6(1H,3H,5H)-trione

| Conditions | Yield |
|---|---|
| In water mixing ratio Fe2(SO4)3*9H2O:alloxan = 1:2 at pH=2; evapd. (water bath); sepn. of crystals; dried; elem.anal.; X-ray diffraction; |
Alloxan Chemical Properties

IUPAC Name: 1,3-diazinane-2,4,5,6-tetrone
Molecular Weight: 142.06968 g/mol
Molecular Formula: C4H2N2O4
Density: 1.681 g/cm3
Melting Point: ~245 °C (dec.)
Boiling Point: 409.5 °C at 760 mmHg
Flash Point: 201.4 °C
Index of Refraction: 1.519
Molar Refractivity: 25.66 cm3
Molar Volume: 84.5 cm3
Polarizability: 10.17*10-24 cm3
Surface Tension: 62.6 dyne/cm
Enthalpy of Vaporization: 76.46 kJ/mol
Vapour Pressure: 2.05E-08 mmHg at 25 °C
XLogP3-AA: -1
H-Bond Donor: 2
H-Bond Acceptor: 4
Tautomer Count: 5
Exact Mass: 142.001457
MonoIsotopic Mass: 142.001457
Topological Polar Surface Area: 92.3
Heavy Atom Count: 10
Complexity: 222
Canonical SMILES: C1(=O)C(=O)NC(=O)NC1=O
InChI: InChI=1S/C4H2N2O4/c7-1-2(8)5-4(10)6-3(1)9/h(H2,5,6,8,9,10)
InChIKey: HIMXGTXNXJYFGB-UHFFFAOYSA-N
EINECS: 200-062-0
Storage temp.: 2-8 °C
Alloxan History
Alloxan (CAS NO.50-71-5) was originally isolated in 1818 by Brugnatelli and got its name in 1838 by Friedrich Wöhler and Justus von Liebig. The name "Alloxan" emerged from an amalgamation of the words "Allantoin" and "Oxalsäure" (oxaluric acid).
Alloxan Production
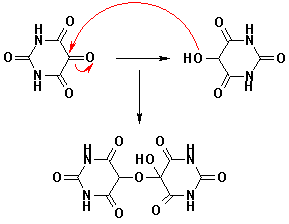
The original preparation for Alloxan (CAS NO.50-71-5) was by oxidation of uric acid by nitric acid. Alloxan is a strong oxidizing agent and it forms a hemiacetal with its reduced reaction product dialuric acid (in which a carbonyl group is reduced to a hydroxyl group) which is called alloxantin.
Alloxan Toxicity Data With Reference
| 1. | eye-rbt 500 mg/24H MLD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,862. | ||
| 2. | cyt-mus-ipr 50 mg/kg | JOHEA8 Journal of Heredity. 65 (1974),345. | ||
| 3. | ipr-mus TDLo:200 mg/kg (female 8D post):TER | JEEMAF Journal of Embryology and Experimental Morphology. 14 (1965),63. | ||
| 4. | orl-rat LD50:5210 mg/kg | 28ZOAH Chemicals in War Prentiss, A.M.,1972,150. | ||
| 5. | ivn-rat LD50:300 mg/kg | CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. 142 (1948),1335. | ||
| 6. | ipr-mus LDLo:300 mg/kg | PSEBAA Proceedings of the Society for Experimental Biology and Medicine. 67 (1948),154. | ||
| 7. | scu-mus LDLo:400 mg/kg | PSEBAA Proceedings of the Society for Experimental Biology and Medicine. 67 (1948),154. | ||
| 8. | ivn-mus LDLo:200 mg/kg | PSEBAA Proceedings of the Society for Experimental Biology and Medicine. 67 (1948),154. | ||
| 9. | ivn-rbt LDLo:300 mg/kg | AJCPAI American Journal of Clinical Pathology. 16 (1946),257. | ||
| 10. | rec-rbt LDLo:180 mg/kg | &nbs |
Alloxan Consensus Reports
Reported in EPA TSCA Inventory.
Alloxan Safety Profile
Poison by intraperitoneal, intravenous, subcutaneous, and rectal routes. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Produces diabetes in experimental animals. Decomposes in storage to release CO2. Do not store in sealed container. Explodes when heated above 170°C. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:  Xn
Xn
Risk Statements: 20/21/22-36/37/38
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed
R36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36: Wear suitable protective clothing
WGK Germany: 3
F: 8-10-23
Hazardous Substances Data: 50-71-5(Hazardous Substances Data)
Alloxan Specification
Alloxan (CAS NO.50-71-5) is also called 2,4,5,6(1H,3H)-Pyrimidinetetrone ; Mesoxalylurea ; Mesoxalylcarbamide ; 4,5,6-Pyrimidinetetrone ; 2,4,5,6-Pyrimidintetron ; 2,4,5,6-Tetraoxohexahydropyrimidine . Alloxan (CAS NO.50-71-5) is a kind of pesticides. It's moderate toxic. It will explosive by heating above 170 °C. Alloxan (CAS NO.50-71-5) is also flammable. It will produce toxic nitrogen oxide fumes when buring. And it decomposits of carbon dioxide at room temperature. So the storage environment should be ventilate, low-temperature and dry. Keep Anthramycin (CAS NO.4803-27-4) separate from raw materials of food. Do not store in a sealed tank.
Related Products
- Alloxan
- Alloxan 5-oxime
- Alloxan monohydrate
- 50715-28-1
- 50715-50-9
- 5071-61-4
- 507-16-4
- 50717-82-3
- 50718-91-7
- 5071-96-5
- 507-19-7
- 507-20-0
- 50720-12-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View