-
Name
Ambroxane
- EINECS 229-861-2
- CAS No. 6790-58-5
- Article Data66
- CAS DataBase
- Density 0.939 g/cm3
- Solubility 1.88mg/L at 20℃
- Melting Point 73-77 °C
- Formula C16H28O
- Boiling Point 273.9 °C at 760 mmHg
- Molecular Weight 236.398
- Flash Point 104.8 °C
- Transport Information
- Appearance white crystals
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Naphtho[2,1-b]furan,dodecahydro-3a,6,6,9a-tetramethyl-, [3aR-(3aa,5ab,9aa,9bb)]-;(-)-Ambrox;(-)-Ambroxan;(-)-Ambroxide;(-)-Norlabdane oxide;Amberlyn Super;Amberlyn Super PM 577;Ambroxide;
- PSA 9.23000
- LogP 4.40800
Synthetic route

-
A
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
B
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With RuCl2(2-(diphenylphosphino)-N-(thiophen-2-ylmethyl)ethanamine)PPh3; hydrogen In toluene at 100℃; under 37503.8 Torr; for 16h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; Inert atmosphere; Autoclave; | A 99.5% B n/a |
| With RuCl2(2-(diphenylphosphino)-N-(2-(methylthio)ethyl)ethanamine)PPh3; hydrogen; sodium methylate In toluene at 100℃; for 16h; Catalytic behavior; Autoclave; | A 67% B 12% |
-
-
320756-17-0
biscyclohomofarnesane-8α,12-diol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 11h; | 98% |

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 10h; | 97% |
-
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With n-butyllithium; p-toluenesulfonyl chloride In tetrahydrofuran; hexane at 0 - 20℃; | 96% |
| With sodium hydride; p-toluenesulfonyl chloride In dichloromethane at 25℃; | 94% |
| With zinc(II) chloride In 1,2-dichloro-ethane for 3h; | 92% |
-
-
1038482-07-3
Δ2(3)-ambrox

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With 10% Pd/C; hydrogen In ethyl acetate at 20℃; for 12h; | 96% |
| With palladium 10% on activated carbon; hydrogen In methanol under 760.051 Torr; | 92% |

-
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; toluene | 95% |
-
-
121067-50-3
<1R-(1α,2β,4aβ,8aα)>-decahydro-1-(2-chloroethyl)-2,5,5,8a-tetramethyl-2-naphthalenol acetate

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; isopropyl alcohol | 92% |
-
-
116163-57-6
(3aS,5aS,9aS,9bR)-3a,6,6,9a-Tetramethyl-decahydro-naphtho[2,1-b]furan-4-one

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine | 90% |

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; zinc(II) iodide In tetrahydrofuran for 1h; Heating; | 85% |
| Multi-step reaction with 2 steps 1: 95 percent / potassium borohydride / ethanol / 10 h / Heating 2: 92 percent / bromotrichloromethane; triphenylphosphine; NaHCO3 / CH2Cl2 / 6 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 97 percent / LiAlH4 / tetrahydrofuran / 3 h / 20 °C 2: 96 percent / n-BuLi; TsCl / hexane; tetrahydrofuran / 0 - 20 °C View Scheme |
-
-
121067-46-7
<1R-(1α,2β,4aβ,8aα)>-1-decahydro-1-(2-iodoethyl)-2,5,5,8a-tetramethyl-2-naphthalenol acetate

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; isopropyl alcohol for 18h; Heating; | 81% |
-
-
31207-73-5
2-((1S,4aS,8aS)-2,5,5,8a-tetramethyl-1,4,4a,5,6, 7,8,8a-octahydronaphthalen-1-yl)ethan-1-ol

-
A
-
31222-15-8
2-[(4aS,8aS)-3,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethylnaphthalen-1-yl]ethanol

-
B
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In nitromethane for 18h; Ambient temperature; | A n/a B 80% |
-
-
31222-15-8
2-[(4aS,8aS)-3,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethylnaphthalen-1-yl]ethanol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With iron(III) chloride In 1,2-dichloro-ethane at 25℃; Reagent/catalyst; enantioselective reaction; | 77% |
| Acidic conditions; |
-
-
41747-05-1
13,14,15,16-tetranor-8βH-labdane-8,12-diol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In nitromethane at 80℃; for 0.5h; | 76% |
| With toluene-4-sulfonic acid In nitromethane at 40℃; for 0.0833333h; | 45% |
-
-
152185-80-3
8α,12-epoxy-13,14,15,16-tetranorlabdan-19-al

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine hydrate In various solvent(s) for 1h; Heating; | 71% |
| With potassium hydroxide; hydrazine hydrate In various solvent(s) for 1h; Heating; | 71% |
-
-
38419-76-0
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-yl acetate

-
A
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
B
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With sodium methylate; carbonic acid dimethyl ester In tetrahydrofuran; methanol at 65℃; for 2h; | A 4% B 67% |
-
-
158577-90-3
<1S-(1α,2β,3β,4β,4aβ,8aα)> decahydro-3-hydroxyl-2,5,5,8a-tetramethylnaphthalene-1-ethanol

-
A
-
6790-58-5
(-)-ambroxide

-
B
-
68365-88-8
8β,12-epoxy-13,14,15,16-tetranorlabdane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 80℃; for 2h; | A 48% B 10% |
-
-
105735-95-3, 105735-96-4
(1R,2R,4aS,8aS)-1-(3-Hydroperoxy-3-methyl-pent-4-enyl)-2,5,5,8a-tetramethyl-decahydro-naphthalen-2-ol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With copper diacetate; iron(II) sulfate In methanol at 50℃; for 3h; | 33% |
| With copper diacetate; iron(II) sulfate In methanol at 50℃; for 3h; Product distribution; other amounts of reagents; | |
| Multi-step reaction with 4 steps 1: triphenylphosphine / diethyl ether / 24 h / 20 °C 2: 83 percent / H2 / 5percent Pd/C / ethanol / 760 Torr / Ambient temperature 3: 1.) aq. NaOCl / 1.) AcOH, CCl4, 0 deg C, 3h, 2.) 30-35 deg C, 3h 4: 80percent NaH / tetrahydrofuran / 3 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 70℃; for 4h; | 20% |
| Multi-step reaction with 2 steps 1.1: potassium permanganate; acetic anhydride / acetone / 2 h / 0 °C 1.2: 97 percent / lithium aluminum hydride / tetrahydrofuran / 2 h / 20 °C 2.1: 92 percent / bromotrichloromethane; triphenylphosphine; NaHCO3 / CH2Cl2 / 6 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1.1: peracetic acid; NaOAc / ethyl acetate / 192 h / 20 °C 2.1: 1 N aq. NaOH / 2-methyl-propan-2-ol / 20 °C 2.2: H2SO4 / ethyl acetate / 2 h / 0 - 20 °C 3.1: 97 percent / NaIO4 / tetrahydrofuran; H2O / 16 h / 20 °C 4.1: 95 percent / peracetic acid / 50 - 55 °C 5.1: 97 percent / LiAlH4 / tetrahydrofuran / 3 h / 20 °C 6.1: 96 percent / n-BuLi; TsCl / hexane; tetrahydrofuran / 0 - 20 °C View Scheme |
-
-
113327-33-6
C20H39N2O3P

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With lithium In various solvent(s) Yield given; |
-
-
113105-33-2
(1R,2R,4aS,8aS)-1-(2-Chloro-ethyl)-2,5,5,8a-tetramethyl-decahydro-naphthalen-2-ol

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 3h; Heating; Yield given; |

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; Yield given; |
-
-
38419-77-1
(4aS,6aR,11aR,11bS)-4,4,6a,11b-Tetramethyl-dodecahydro-7,9-dioxa-cyclohepta[a]naphthalene

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane |

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With naphthalene-2-sulfonate Erhitzen unter vermindertem Druck; |
-
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
A
-
31207-73-5
2-((1S,4aS,8aS)-2,5,5,8a-tetramethyl-1,4,4a,5,6, 7,8,8a-octahydronaphthalen-1-yl)ethan-1-ol

-
B
-
31222-15-8
2-[(4aS,8aS)-3,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethylnaphthalen-1-yl]ethanol

-
C
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane for 0.5h; Heating; Title compound not separated from byproducts; | A 7.2 % Chromat. B 16.8 % Chromat. C 67.4 % Chromat. |
| With toluene-4-sulfonic acid In dichloromethane at 20℃; for 1h; Title compound not separated from byproducts; | A 7.6 % Chromat. B 16.8 % Chromat. C 65.2 % Chromat. |

-
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-
A
-
31222-15-8
2-[(4aS,8aS)-3,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethylnaphthalen-1-yl]ethanol

-
B
-
6790-58-5
(-)-ambroxide

-
C
-
68365-88-8
8β,12-epoxy-13,14,15,16-tetranorlabdane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane for 30h; Heating; Title compound not separated from byproducts; | A 11.2 % Chromat. B 29.6 % Chromat. C 59.1 % Chromat. |


| Conditions | Yield |
|---|---|
| Stage #1: (E,E)-homofarnesyl triethylsilyl ether With (R)-2-(o-fluorobenzyloxy)-2'-methoxy-1,1'-binaphthyl*SnCl4 In toluene at -78℃; for 72h; Stage #2: With 1H-imidazole; triethylsilyl chloride In N,N-dimethyl-formamide at 20℃; for 5h; Stage #3: With TFA*SnCl4 In toluene at -78℃; for 24h; Further stages. Title compound not separated from byproducts.; |

| Conditions | Yield |
|---|---|
| With naphthalene-2-sulfonate Erhitzen unter vermindertem Druck; |
-
-
16736-51-9
8-hydroxy-15,16-dinor-labdan-13-one

-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 83 percent / sodium chromate; acetic anhydride; glacial acetic acid / benzene / 4 h / 70 °C 2: 95 percent / potassium borohydride / ethanol / 10 h / Heating 3: 92 percent / bromotrichloromethane; triphenylphosphine; NaHCO3 / CH2Cl2 / 6 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 83 percent / sodium chromate; acetic anhydride; glacial acetic acid / benzene / 4 h / 70 °C 2: 85 percent / zinc iodide; sodium borohydride / tetrahydrofuran / 1 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 96 percent / PhLi / tetrahydrofuran; cyclohexane; diethyl ether / 1 h / 20 °C 2.1: tetrahydrofuran / 24 h / 20 °C 3.1: H2O2; NaOH / tetrahydrofuran 4.1: 100 percent / imidazole / dimethylformamide / 20 °C 5.1: (R)-2-(o-fluorobenzyloxy)-2'-methoxy-1,1'-binaphthyl*SnCl4 / toluene / 72 h / -78 °C 5.2: ClSiEt3; imidazole / dimethylformamide / 5 h / 20 °C 5.3: TFA*SnCl4 / various solvent(s); toluene / 24 h / -78 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sodium benzoate; [Ir(2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine)2(4,4'-di-tert-butyl-2,2'-bipyridine)](PF6) In acetonitrile at 20℃; for 2h; Inert atmosphere; Irradiation; regioselective reaction; | 95% |
-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; C17H18ClFeN6O6(2-); 3-chloro-benzenecarboperoxoic acid In water; acetonitrile at 20℃; | 90% |
| With (R,R)-Fe(PDP); dihydrogen peroxide; acetic acid regioselective reaction; | 80% |
| With 2,6-dichloropyridine N-oxide; dichloro(5,10,15,20-tetramesitylporphyrinato)ruthenium(IV) In 1,2-dichloro-ethane at 40℃; for 24h; Reagent/catalyst; Inert atmosphere; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With benzophenone In acetonitrile at 20℃; for 2h; Inert atmosphere; UV-irradiation; stereoselective reaction; | 89% |
| With benzophenone In acetonitrile at 20℃; for 2h; Inert atmosphere; UV-irradiation; chemoselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With [Ir(dF(CF3)ppy)2(5,5'dCF3bpy)]PF6; dibutylphosphoric acid tetrabutylammonium salt In dichloromethane at 20℃; for 24h; Glovebox; Inert atmosphere; Irradiation; | 88% |
| With 2-chloroanthracene-9,10-dione In dichloromethane at 20℃; for 4h; Irradiation; Optical yield = 48 %de; diastereoselective reaction; |
-
-
6790-58-5
(-)-ambroxide

| Conditions | Yield |
|---|---|
| With 4-toluenesulfonyl azide; 4-Benzoylpyridine In benzene at 20℃; for 3h; UV-irradiation; Inert atmosphere; chemoselective reaction; | 77% |

| Conditions | Yield |
|---|---|
| With pentacene-5,7,12,14-tetraone; potassium carbonate In dichloromethane at 20℃; for 24h; Inert atmosphere; Irradiation; | 74% |
-
-
6790-58-5
(-)-ambroxide

-
-
308255-94-9
(1S,6S)-2-(2-bromoethyl)-1,3,7,7-tetramethylbicyclo[4.4.0]dec-2-ene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; tetraethylammonium bromide In dichloromethane at 20℃; Ring cleavage; | 72% |

| Conditions | Yield |
|---|---|
| With 4-Benzoylpyridine In tetrahydrofuran at 20℃; for 6h; Inert atmosphere; UV-irradiation; | 70% |
-
-
6790-58-5
(-)-ambroxide

-
-
308255-96-1
(4aS,8aS)-5-(2-iodoethyl)-1,1,4a,6-tetramethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalene

| Conditions | Yield |
|---|---|
| With aluminium trichloride; sodium iodide In dichloromethane; acetonitrile at 20℃; Ring cleavage; | 69% |

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); 5,5'-dimethyl-2,2'-bipyridine; (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone; sodium carbonate In benzene at 20℃; for 96h; Schlenk technique; diastereoselective reaction; | A 69% B n/a |
| With bis(acetylacetonate)nickel(II); 5,5'-dimethyl-2,2'-bipyridine; (4-methoxyphenyl)(4-(trifluoromethyl)phenyl)methanone; sodium hydrogencarbonate In benzene at 20℃; for 96h; Schlenk technique; Inert atmosphere; Irradiation; Overall yield = 69 %; Overall yield = 76.7 mg; diastereoselective reaction; | A n/a B n/a |


| Conditions | Yield |
|---|---|
| With eosin In tert-butyl alcohol at 0 - 60℃; for 48h; Sealed tube; Inert atmosphere; Irradiation; | 68% |
-
-
6790-58-5
(-)-ambroxide

-
-
1217488-90-8
C16H28O3

| Conditions | Yield |
|---|---|
| With Botrytis cynerea for 72h; Microbiological reaction; chemoselective reaction; | 60% |
-
-
6790-58-5
(-)-ambroxide

-
A
-
13456-36-5
(1R,2R,4aS,8aS)-(2-hydroxy-2,5,5,8a-tetramethylperhydronaphthyl)acetic acid

| Conditions | Yield |
|---|---|
| With [((S,S)-N,N′-bis(2-pyridylmethyl)-(S,S)-2,2′-bipyrrolidine)FeII(OTf)2]; dihydrogen peroxide; acetic acid In water; acetonitrile at 0 - 20℃; Catalytic behavior; Inert atmosphere; regiospecific reaction; | A 29% B 54% |

| Conditions | Yield |
|---|---|
| With ethanol; bis-[(trifluoroacetoxy)iodo]benzene In dimethyl sulfoxide; 1,2-dichloro-ethane at 20℃; for 12h; Schlenk technique; Inert atmosphere; Irradiation; diastereoselective reaction; | 53% |
Ambroxane Chemical Properties
Molecular Structure of Ambroxide (CAS NO. 6790-58-5):
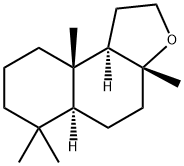
EINECS: 229-861-2
Systematic Name: 3a,6,6,9a-Tetramethyl-2,4,5,5a,7,8,9,9b-octahydro-1H-benzo[e]benzofuran
Molecular Formula: C16H28O
Molecular Weight: 236.3929
SMILES: O2C1(CCC3C(C1CC2)(C)CCCC3(C)C)C
InChI: InChI=1/C16H28O/c1-14(2)8-5-9-15(3)12(14)6-10-16(4)13(15)7-11-17-16/h12-13H,5-11H2,1-4H3
InChIKey: YPZUZOLGGMJZJO-UHFFFAOYAI
Index of Refraction: 1.48
Molar Refractivity: 71.59 cm3
Molar Volume: 251.6 cm3
Surface Tension: 34.6 dyne/cm
Density: 0.939 g/cm3
Flash Point: 104.8 °C
Enthalpy of Vaporization: 49.16 kJ/mol
Boiling Point: 273.9 °C at 760 mmHg
Vapour Pressure: 0.00934 mmHg at 25 °C
Melting Point: 74-76 °C(lit.)
Water Solubility: 2.436 mg/L at 25 °C
Alpha: -30 º (c=1% in toluene )
Ambroxane Safety Profile
Safety Information of Ambroxide (CAS NO. 6790-58-5):
WGK Germany: 1
Ambroxane Specification
Ambroxide with cas registry number of 6790-58-5 is colorless to white crystalline solid, known as 8alpha,12-Oxido-13,14,15,16-tetranorlabdane ; Dodecahydro-3a,6,6,9a-tetramethylnaphtho(2,1-b)furan, (3aR-(3aalpha,5abeta,9aalpha,9bbeta))- ; FEMA No. 3471 ; Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR-(3aalpha,5abeta,9aalpha,9bbeta))- ; (3AR-(3aalpha,5abeta,9aalpha,9bbeta))-dodecahydro-3a,6,6,9a-tetramethylnaphtho(2,1-b)furan ; Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)- ; 1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(sup 4,9))tridecane . It is used in flavor of advanced perfumes and cosmetics.
Related Products
- Ambroxane
- 67905-90-2
- 67906-09-6
- 67906-37-0
- 67907-14-6
- 67908-96-7
- 67909-31-3
- 67909-49-3
- 67910-12-7
- 67911-21-1
- 679-13-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View