-
Name
Copper(I) iodide
- EINECS 231-674-6
- CAS No. 7681-65-4
- Article Data243
- CAS DataBase
- Density 5.62 g/cm3
- Solubility insoluble in water
- Melting Point 605 °C(lit.)
- Formula CuI
- Boiling Point 1290 °C
- Molecular Weight 190.451
- Flash Point 1290°C
- Transport Information UN 3077 9/PG 3
- Appearance white to grey or tan powder
- Safety 22-24/25-26-61
- Risk Codes 22-36/37/38-50/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N,
N, Xi
Xi
- Synonyms Coppermonoiodide;Copper(1+) iodide;Copper(I) iodide;Cuprous iodide(CuI);Copper iodide (CuI);
- PSA 0.00000
- LogP -2.99850
Synthetic route

-
-
201230-82-2
carbon monoxide

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
A
-
7681-65-4
copper(l) iodide

-
B
-
13463-39-3, 71564-36-8
tetracarbonyl nickel

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; in autoclave at 200 atm and 200 - 250°C;; | A n/a B 100% |
| In neat (no solvent) High Pressure; in autoclave at 120 - 180°C;; | A n/a B 100% |

-
-
279221-52-2
SmI2(O(CH2)4)4

-
-
35342-67-7, 35342-68-8
copper (I) tert-butoxide

-
A
-
7681-65-4
copper(l) iodide

-
C
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

| Conditions | Yield |
|---|---|
| In tetrahydrofuran all operations in sealed evacuated tubes with thoroughly dried and degassed solvents; soln. of t-BuOCu added to a blue-green soln. of Sm complex; ppt. sepd. and washed with 10% HNO3; ppt. of CuI insoluble in HNO3 sepd. off; remaining soln. concd. and kept at 80° until complete dissolution of a fine ppt.; cooled slowly to ca. 20°; crystal sepd.; dried; identified by elem. anal., IR; | A 100% B 97.7% C 92.3% |

-
-
147312-03-6
LaI3(tetrahydrofuran)3

-
-
35342-67-7, 35342-68-8
copper (I) tert-butoxide

-
A
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran all operations in sealed evacuated tubes with thoroughly dried and degassed solvents; soln. of the cuprate added to a soln. of La complex; stirred for 0.5 h at ca. 20° and for 2 h at 60°; ppt. of CuI sepd. by centrifugation; reaction mixt. concd. and kept at 80° until complete dissolution of a finely dispersed ppt.; cooledslowly to 20°; crystals sepd. and dried in vacuo; identified by elem. anal. and IR spectrum; | A 90.2% B 95% |

-
-
113316-94-2
tris(tetrahydrofurane)triiodidoneodymium(III)

-
-
35342-67-7, 35342-68-8
copper (I) tert-butoxide

-
A
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran all operations in sealed evacuated tubes with thoroughly dried and degassed solvents; soln. of the cuprate added to a soln. of Nd complex; stirred for 0.5 h at ca. 20° and for 2 h at 60°; ppt. of CuI sepd. by centrifugation; reaction mixt. concd. and kept at 80° until complete dissolution of a finely dispersed ppt.; cooledslowly to 20°; crystals sepd. and dried in vacuo; identified by elem. anal. and IR spectrum; | A n/a B 90.3% |

-
-
7553-56-2
iodine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In acetone | |
| In neat (no solvent) on treatment with I2-vapors at 360°C;; | |
| vapor deposited Cu film was inserted into an iodine-containing reacted atmosphere;; |

| Conditions | Yield |
|---|---|
| In water pptg. from a CuCl2*2H2O soln. with an excess of KI soln.; Debye-Scherrer XRD; |

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In not given iodine containing compounds used; | |
| With alkali sulfite; copper(II) sulfate In not given iodine containing compounds used; |

| Conditions | Yield |
|---|---|
| In water; xylene byproducts: CuBr2; shaking a soln. of CuBr in concd. Br(1-) soln. with I2 in xylol, reversible react.;; |

| Conditions | Yield |
|---|---|
| In water; xylene byproducts: CuCl2; shaking a soln. of CuCl in concd. Cl(1-) soln. with I2 in xylol, reversible react.;; |

| Conditions | Yield |
|---|---|
| In ethanol Kinetics; alcoholic soln. of iodine addn. to AgCl in container with ground stopper, keeping for 2, 4 or 8 h at 60°C; X-ray diffraction; |


| Conditions | Yield |
|---|---|
| In acetone on addn. of a soln. of excess I2 acetone;; |

-
-
7553-56-2
iodine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
7681-11-0
potassium iodide

-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In water Kinetics; |


| Conditions | Yield |
|---|---|
| in presence of Cu(2+); |
-
-
7553-56-2
iodine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
7439-98-7
molybdenum

-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) byproducts: Cu(y)MoO(3-z); molar ratio Cu:Mo:O:I 3:8:23:3, heating (48 h, 500°C, vac.); X-ray diffraction; |

-
-
7553-56-2
iodine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
7439-98-7
molybdenum

-
B
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) molar ratio Cu:Mo:O:I 3:12:31:3, heating (48 h, 500°C, vac.); X-ray diffraction; |

-
-
7553-56-2
iodine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
A
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| 300-340 K, total pressure E-3 Torr; monitored by laser-induced fluorescence spectroscopy; |


| Conditions | Yield |
|---|---|
| In ethanol Kinetics; reaction of alcoholic soln. of iodine with CuF2 at 60°C; X-ray diffraction; |

| Conditions | Yield |
|---|---|
| With sulfur dioxide In water Cu salts from Cu minerals;; |
-
-
7553-56-2
iodine

-
-
7789-45-9
copper(ll) bromide

-
A
-
7681-65-4
copper(l) iodide

-
B
-
7787-70-4
copper(I) bromide

| Conditions | Yield |
|---|---|
| In ethanol Kinetics; reaction of alcoholic soln. of iodine with CuBr2 at 60°C; X-ray diffraction; |


| Conditions | Yield |
|---|---|
| In ethanol Kinetics; reaction of alcoholic soln. of iodine with CuCl2 at 60°C; X-ray diffraction; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: aldehyde, O2, ethylene, methane, hydrocarbons; at about 310°C in aCO2-atmoshere;; | |
| In neat (no solvent) on treatment of CuO with gaseous methyl iodide;; |
-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
74-88-4
methyl iodide

-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; byproducts: CH3; Ar carrier gas; heating (300 - 696 K); not isolated; |
-
-
7790-99-0
Iodine monochloride

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
A
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: I2, CuCl2;; High Pressure; | |
| In neat (no solvent) byproducts: I2, CuCl2;; High Pressure; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) on heating to 400-500°C;; | |
| In neat (no solvent) on heating to 400-500°C;; |


| Conditions | Yield |
|---|---|
| Kinetics; solid body react. under N2 at 200-300°C; | |
| Kinetics; solid body react. in vac.at 200-300°C; | |
| Kinetics; solid body react. under N2 at 200-300°C; | |
| Kinetics; solid body react. in vac.at 200-300°C; |


| Conditions | Yield |
|---|---|
| In hydrogenchloride pptn. with KI;; | |
| In water pptn. from the soln. of CuCl in aq. NH4Cl with KI;; | >99 |
| In water react. of CuCl with KI-soln. with passing through of N2; washing with alcohol on a filter, drying in N2 flow and then in vacuum; | |
| In hydrogenchloride |

| Conditions | Yield |
|---|---|
| In water formation of crystals;; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydroxylamine hydrochloride; gelatin In water byproducts: N2O, KCl; NH2OH*HCl, KI added rapidly to gelatin stabilized CuO suspn. with stirring for 5 h under agitation at room temp., pH adjusted to 8-9 with HCl; washed thoroughly, vacuum dried at room temp.; |
-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In diethyl ether; acetonitrile at 20℃; for 2h; | 100% |
-
-
7681-65-4
copper(l) iodide

-
-
143-66-8
sodium tetraphenyl borate

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane Irradiation (UV/VIS); under Ar; mole ratio NaBPh4 : CuI = 1 : 1; irradn. (254 nm) for 1 h gave deposition of Cu; deposit sepd., washed with acetone and water, and dried in vac. to give pure Cu; | 100% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran; diethyl ether under N2, etheral soln. of MeLi was added to a stirred suspn. of CuI in THF at -10°C, HMPT was also added, stirred for 30 min at 0°C; not isolated; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 48℃; Inert atmosphere; | 100% |
| In acetonitrile dissolving CuI and NPr4I in MeCN, concg.; |

| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile at 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; tert-butyl methyl ether at 10 - 48℃; for 0.75h; Inert atmosphere; | 100% |
| In tetrahydrofuran at 50℃; Inert atmosphere; | 600 g |
| Stage #1: copper(l) iodide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran at 50℃; Inert atmosphere; Stage #2: In tert-butyl methyl ether at 6℃; for 1h; Inert atmosphere; | 600 g |
-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 18h; Inert atmosphere; Schlenk technique; stereoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: copper(l) iodide; lead(II) nitrate; potassium iodide In water for 2h; Stage #2: at 620℃; for 72h; Sealed tube; | 100% |

-
-
7681-65-4
copper(l) iodide

-
-
126215-85-8
5-chloro-2-(pent-4-yn-1-yl)pyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |
-
-
7681-65-4
copper(l) iodide

-
-
1380612-64-5
2,6-bis(5-(tert-butyl)-1H-pyrazol-3-yl)pyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 24h; Inert atmosphere; Schlenk technique; | 100% |
-
-
7681-65-4
copper(l) iodide

-
-
591215-89-3
[((p-tert-butyl)C6H4)2B(CH2P(phenyl)2)2][5-azoniaspiro[4.4]nonane]

-
-
820219-08-7
[[(p-(t)BuPh)2B(CH2PPh2)2]CuI][5-azonia-spiro[4.4]nonane]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; ethanol equiv. amt. of solid CuI and B-compd. were mixed in THF:EtOH=4:0.3, stirring for 2.5 h; volatiles were removed in vac., solid was washed with Et2O, dried in vac., elem. anal.; | 99.4% |
-
-
7681-65-4
copper(l) iodide

-
-
345964-32-1
6-bromo-2,2,4,4-tetramethyl chroman-8-carbaldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
607714-16-9
6-Trimethylsilanylethynyl-2,2,4,4-tetramethyl chroman-8-carbaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine; dichlorobis(triphenylphosphine)palladium[II] In tetrahydrofuran; hexane | 99% |

-
-
7681-65-4
copper(l) iodide

-
-
607713-99-5
ethyl 8-iodo-2,2,4,4-tetramethyl-chroman-6-carboxylate

-
-
1066-54-2
trimethylsilylacetylene

-
-
607714-00-1
Ethyl-8-trimethylsilanyl-ethynyl-2,2,4,4-tetramethyl chroman-6-carboxylate

| Conditions | Yield |
|---|---|
| dichlorobis(triphenylphosphine)palladium[II] In hexane; triethylamine | 99% |


| Conditions | Yield |
|---|---|
| In diethyl ether at -78 - -35℃; for 0.333333h; Inert atmosphere; | 99% |
| In tetrahydrofuran Ar, ratio CuI:CH3Li = 1:2; | |
| In hexane |

| Conditions | Yield |
|---|---|
| With KI In water pyridine was added to stirred soln. of CuI in concd. aq. KI; filtered; washed (satd. aq. KI, H2O, MeOH, hexane); recrystd. (CH2Cl2/pentane); dried (vac.); | 99% |
| In ethanol at 20℃; for 0.5h; Sealed tube; | 80% |
| With potassium iodide In water |
-
-
7681-65-4
copper(l) iodide

-
-
359-37-5
Iodotrifluoroethylene

-
-
7440-43-9
cadmium

-
-
102682-87-1
trifluorovinyl copper

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide byproducts: CdI2; N2; slight excess of acid-washed Cd powder added to alkene soln.; mild exothermic reaction after induction period; removal of excess Cd by press. filtrn. under N2; stirring mixt. at 0 °C for half h with CuI or CuBr; final warming to room temp.; (19)F NMR; | 99% |

-
-
7681-65-4
copper(l) iodide

| Conditions | Yield |
|---|---|
| In not given treated at room temp. for 24 h; further intermediate; followed by UV and by ESI-MS; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane piperidine was dissolved in CH2Cl2 under N2 at 25°C; CuI was added; mixt. was stirred under N2 until CuI dissolved; | 99% |
| With potassium iodide In acetone excess of ligand in acetone soln. is added to a stirred soln. of CuI in concd. aq. KI soln., soln. kept standing for a few min, pptn.; filtn., washing with satd. aqueous KI, water, MeOH and hexanes, product is dried in vac.; | >99 |
| With potassium iodide In water |

| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile cyclodiphosphazane treated with 2 equiv. of CuI in CH2Cl2/MeCN (1/1); | 99% |


| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile cyclodiphosphazane treated with 2 equiv. of CuI in CH2Cl2/MeCN (1/1); | 99% |

-
-
96-45-7
N,N'-ethylenethiourea

-
-
7681-65-4
copper(l) iodide

-
-
1094209-90-1
tris(iodobis(ethylenethiourea)copper(I))

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide CuI, ethylenethiourea and few drops DMF were ground together for severalminutes; DMF was evapd.; | 99% |
| In water CuI and ethylenethiourea in water were heated to boiling; supernatant was decanted while hot and cooled, product was collected andwashed with water; elem. anal.; | 50% |
-
-
7681-65-4
copper(l) iodide

-
-
443287-45-4
triacetylacetonatotripyridinetetrasulfido trimolybdenum hexafluorophosphate

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile; benzene stoich., CuI added to soln. of Mo complex in CH2Cl2, evapd., dissolved (CH3CN), benzene deposited; crystd. for 3 d, elem. anal.; | 99% |

-
-
7681-65-4
copper(l) iodide

-
-
161265-03-8
4,5-bis(diphenylphosphino)-9,9-dimethylxanthene

-
-
1218788-80-7
Cu[9,9-dimethyl-4,5-bis(diphenylphosphine)xanthene]I

| Conditions | Yield |
|---|---|
| In acetonitrile | 99% |
| In CH2Cl2 | 99% |
| In acetonitrile at 50℃; for 2h; | 99% |
-
-
7681-65-4
copper(l) iodide

-
-
776315-37-8
1,2-bis(diphenylphosphino)-1′-(diisopropylphosphino)-4-tert-butylferrocene

-
-
1231929-92-2
P,P',P''-[1,2-bis(diphenylphosphino)-1'-(diisopropylphosphino)-4-tert-butylferrocene]iodocopper(I)

| Conditions | Yield |
|---|---|
| In acetonitrile (Ar) to soln. triphosphine in MeCN CuI was added, refluxed for 3 h; react. mixt. was cooled, solvent was removed in vacuo; elem. anal.; | 99% |
-
-
7681-65-4
copper(l) iodide

-
-
1159850-42-6
1-diphenylphosphino-2-diphenylphosphino-4-tert-butyl-cyclopentadienyl-1'-diphenylphosphino-3'-tert-butyl-cyclopentadienyliron

-
-
1231929-91-1
P,P',P''-[1,1',2-tris(diphenylphosphino)-3',4-di-tert-butylferrocene]iodocopper(I)

| Conditions | Yield |
|---|---|
| In acetonitrile (Ar) to soln. triphosphine in MeCN CuI was added, refluxed for 3 h; react. mixt. was cooled, solvent was removed in vacuo; elem. anal.; | 99% |
-
-
7681-65-4
copper(l) iodide

-
-
13648-83-4
bis(diethylamino)-2,3,4,5,6-pentafluorophenylphosphane

-
-
1311311-97-3
bis[bis(diethylamino)(pentafluorophenyl)phosphate]iodidocopper(I)

| Conditions | Yield |
|---|---|
| In chloroform-d1 inert gas; CuI (0.36 mmol) added to soln. of ligand (0.70 mmol), mixt. stirred at room temp. for 30 min; filtered, evapd.; | 99% |
-
-
7681-65-4
copper(l) iodide

-
-
28791-97-1
tris(3,5-dimethylpyrazolyl)methane

-
-
1392097-26-5
(tris(3,5-dimethyl-1-pyrazolyl)methane)CuI

| Conditions | Yield |
|---|---|
| In acetonitrile under N2; soln. of ligand in MeCN added to stirred soln. of CuI in MeCN;stirred for 2 h; filtered; ppt. dried under vac.; elem. anal.; | 99% |
| at 20℃; |
CUPROUS IODIDE Chemical Properties
Molecular Formula:CuI
Molecular Weight:190.45
EINECS:231-674-6
Density:5.62 g/mL at 25 °C(lit.)
Melting Point:605 °C
Boiling Point:1290 °C
Solubility:Insoluble in water
Sensitive:Air & Moisture Sensitive
Appearance:Off white powder
Cuprous iodide's(7681-65-4) Synonyms: Copper iodide (CuI);CuI;Hydro-giene;Marshite;COPPER(+1)IODIDE;COPPER IODIDE;COPPER(I) IODIDE;Ciras
Cuprous iodide's(7681-65-4) Molecular Structure:
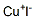
CUPROUS IODIDE Uses
CUPROUS IODIDE Safety Profile
When heated to decomposition it emits toxic fumes of I−.
Safety Information of Cuprous iodide(7681-65-4)
Hazard Codes Xn,N,Xi
Risk Statements 22-36/37/38-50/53
Safety Statements 22-24/25-26-61
RIDADR UN 3077 9/PG 3
WGK Germany 3
F 8
HS Code 28276000
CUPROUS IODIDE Standards and Recommendations
CUPROUS IODIDE Specification
Stability and Reactivity of Cuprous iodide(7681-65-4)
【Disposal Code】15
【Incompatibilities】Oxidizing agents, alkali metals, potassium, acetylene.
【Stability】Stable under normal temperatures and pressures.
【Decomposition】Hydrogen iodide, iodine.
【Combustion Products】Fire may produce irritating, corrosive and/or toxic gases.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View