-
Name
Kresoxim-methyl
- EINECS 604-351-6
- CAS No. 143390-89-0
- Article Data16
- CAS DataBase
- Density 1.1 g/cm3
- Solubility 2 mg/L (20 °C ) in water
- Melting Point 98-100 °C
- Formula C18H19NO4
- Boiling Point 429.4 °C at 760 mmHg
- Molecular Weight 313.353
- Flash Point 171.2 °C
- Transport Information UN 3077 9/PG 3
- Appearance
- Safety 2-36/37-60-61
- Risk Codes 40-50/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms Bas 490F;Methyl (E)-alpha-(methoxyimino)-2-((2-methylphenoxy)methyl)benzeneacetate;BAS 490 F;Benzeneacetic acid,R-(methoxyimino)-2-[(2- methylphenoxy)methyl]-,methyl ester,(RE)-;Methyl (E)-2-methoxyimino-(2-(o-tolyloxymethyl)Phenyl)Acetate;Kresoxim methyl;
- PSA 57.12000
- LogP 3.09750
Synthetic route
-
-
593-56-6
N-methoxylamine hydrochloride

-
-
143211-10-3
2-oxo-2-(((o-tolyl)oxymethyl)phenyl)acetic acid methyl ester

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| In methanol for 6h; Reflux; | 84.8% |
-
-
95-48-7
ortho-cresol

-
-
133409-72-0
methyl (E)-2-[2-(bromomethyl)phenyl]-2-(methoxyimino)acetate

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With copper(l) iodide In butanone for 4h; Concentration; Reagent/catalyst; Reflux; | 80% |
-
-
593-56-6
N-methoxylamine hydrochloride

-
-
143211-10-3
2-oxo-2-(((o-tolyl)oxymethyl)phenyl)acetic acid methyl ester

-
A
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

-
B
-
248582-68-5
methyl (Z)-O-methyloximino-2-[(2-methyl)phenoxymethyl]phenylacetate

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 17.5h; Condensation; | A 63% B 28% |

-
-
248582-68-5
methyl (Z)-O-methyloximino-2-[(2-methyl)phenoxymethyl]phenylacetate

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With iodine In benzene at 63℃; for 120h; Isomerization; UV-irradiation; |

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 61 percent / Pd(PPh3)4 / tetrahydrofuran / 4 h / 0 °C 2: 63 percent / pyridine / 17.5 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 61 percent / Pd(PPh3)4 / tetrahydrofuran / 4 h / 0 °C 2: 28 percent / pyridine / 17.5 h / 20 °C 3: I2 / benzene / 120 h / 63 °C / UV-irradiation View Scheme |
-
-
143211-10-3
2-oxo-2-(((o-tolyl)oxymethyl)phenyl)acetic acid methyl ester

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 28 percent / pyridine / 17.5 h / 20 °C 2: I2 / benzene / 120 h / 63 °C / UV-irradiation View Scheme |
-
-
143390-88-9
methyl 2-(2-methylphenoxymethyl)phenylglyoxylate dimethyl acetal

-
-
593-56-6
N-methoxylamine hydrochloride

-
-
143211-10-3
2-oxo-2-(((o-tolyl)oxymethyl)phenyl)acetic acid methyl ester

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; dichloromethane |

-
-
150869-72-0
methyl E-2-(2-methylphenoxymethyl)phenylglyoxylate oxime

-
-
74-87-3
methylene chloride

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With sodium methylate In 1-methyl-pyrrolidin-2-one; methanol; diethyl ether |
-
-
189813-45-4
(E)-methyl 2-(2-(chloromethyl)phenyl)-2-(methoxyimino)acetate

-
-
95-48-7
ortho-cresol

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 50℃; for 64h; |
-
-
108475-90-7
2-[(2-methylphenoxy)methyl]benzoic acid

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: thionyl chloride / 3 h / Reflux 2.1: tetrabutylammomium bromide / toluene / 2.5 h / 20 - 35 °C 3.1: hydrogenchloride; acetic anhydride / tert-butyl methyl ether / 10 h / -5 - 30 °C 3.2: 5 h / Reflux 4.1: methanol / 6 h / Reflux View Scheme |
-
-
143211-21-6
2-[(2-methylphenoxy)methyl]benzoyl chloride

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: tetrabutylammomium bromide / toluene / 2.5 h / 20 - 35 °C 2.1: hydrogenchloride; acetic anhydride / tert-butyl methyl ether / 10 h / -5 - 30 °C 2.2: 5 h / Reflux 3.1: methanol / 6 h / Reflux View Scheme |
-
-
143211-11-4
2-[(2-methylphenoxy)methyl]benzoyl cyanide

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogenchloride; acetic anhydride / tert-butyl methyl ether / 10 h / -5 - 30 °C 1.2: 5 h / Reflux 2.1: methanol / 6 h / Reflux View Scheme |
-
-
67-56-1
methanol

-
-
137169-29-0
2-methoxyimino(2-(o-tolyl)oxymethylphenyl)acetic acid

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2-methoxyimino(2-(o-tolyl)oxymethylphenyl)acetic acid With thionyl chloride In N,N-dimethyl-formamide at 40 - 45℃; for 8h; Stage #2: methanol at 60 - 65℃; for 2h; | 42.5 g |
-
-
95-48-7
ortho-cresol

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium methylate / methanol / 1 h / 20 °C 1.2: 2 h / 190 °C 2.1: thionyl chloride / 3 h / Reflux 3.1: tetrabutylammomium bromide / toluene / 2.5 h / 20 - 35 °C 4.1: hydrogenchloride; acetic anhydride / tert-butyl methyl ether / 10 h / -5 - 30 °C 4.2: 5 h / Reflux 5.1: methanol / 6 h / Reflux View Scheme |
-
-
107960-33-8
2-methyl-α-hydroxyiminobenzeneacetonitrile

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 11 - 15 °C 2.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 2.2: 25 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 11 - 15 °C 2.1: sodium hydroxide / water / 3 h / 70 - 75 °C 2.2: 5 - 10 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 1 h / 7 - 15 °C 2.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 2.2: 25 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme |
-
-
178983-50-1
2-methyl-a-cyanobenzoxime potassium

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 2.2: 25 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium hydroxide / water / 3 h / 70 - 75 °C 2.2: 5 - 10 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium carbonate / water / 1.6 h / 80 - 85 °C 2.2: 6 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme |

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 2.2: 25 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium hydroxide / water / 3 h / 70 - 75 °C 2.2: 5 - 10 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 2.1: sodium carbonate / water / 1.6 h / 80 - 85 °C 2.2: 6 °C 3.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 4.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium methylate / methanol / 1 h / 20 °C 1.2: 2 h / 190 °C 2.1: thionyl chloride / 3 h / Reflux 3.1: tetrabutylammomium bromide / toluene / 2.5 h / 20 - 35 °C 4.1: hydrogenchloride; acetic anhydride / tert-butyl methyl ether / 10 h / -5 - 30 °C 4.2: 5 h / Reflux 5.1: methanol / 6 h / Reflux View Scheme |
-
-
22364-68-7
2-tolylmethylnitrile

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: ethyl nitrite; sodium hydroxide / chlorobenzene / 1 h / 15 °C 2.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 3.1: sodium carbonate / water / 1.6 h / 80 - 85 °C 3.2: 6 °C 4.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 5.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: isopentyl nitrite; potassium hydroxide / chlorobenzene / 1 h / 15 °C 2.1: sodium hydroxide / water / 0.5 h / 8 - 10 °C 3.1: sodium carbonate / water / 1.6 h / 80 - 85 °C 3.2: 6 °C 4.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 5.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium carbonate; n-propyl nitrite / dichloromethane / 1.5 h / 5 °C 2.1: sodium hydroxide / water / 0.5 h / 11 - 15 °C 3.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 3.2: 25 °C 4.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 5.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme |

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium carbonate / water / 1.6 h / 80 - 85 °C 1.2: 6 °C 2.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 3.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / water / 2.5 h / 50 - 65 °C 1.2: 25 °C 2.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 3.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / water / 3 h / 70 - 75 °C 1.2: 5 - 10 °C 2.1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 3.1: copper(l) iodide / butanone / 4 h / Reflux View Scheme |
-
-
120974-97-2
methyl-(E)-2-(2-tolyl)-2-methoxyiminoacetate

-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dibenzoyl peroxide; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / 1,2-dichloro-ethane / 4 h / 55 °C 2: copper(l) iodide / butanone / 4 h / Reflux View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| With Iodine monochloride In dichloromethane; acetic acid at 20℃; for 5h; | 95% |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

-
-
1007364-30-8
(E)-2-methoxyimino-2-{[2-(2-tolyl)oxymethyl]phenyl}acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate With sodium hydroxide In water; toluene at 40 - 70℃; for 6h; Stage #2: With hydrogenchloride In water; toluene at 10 - 15℃; pH=1.5; | 93.75% |
| With water; sodium hydroxide In methanol for 3h; Reflux; | 92% |
| With sodium hydroxide In methanol at 65℃; | 86% |
| With sodium hydroxide; water In methanol Reflux; |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

-
-
156996-22-4
N-Methyl-E-2-methoxyimino-2-[(2-methylphenyloxymethyl)phenyl]-acetamide

| Conditions | Yield |
|---|---|
| In methylamine | |
| In methylamine |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Iodine monochloride / dichloromethane; acetic acid / 5 h / 20 °C 2: copper(l) iodide; bis-triphenylphosphine-palladium(II) chloride; triethylamine / N,N-dimethyl-formamide / 3 h / 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Iodine monochloride / dichloromethane; acetic acid / 5 h / 20 °C 2: copper(l) iodide; bis-triphenylphosphine-palladium(II) chloride; triethylamine / N,N-dimethyl-formamide / 3 h / 20 °C / Inert atmosphere 3: Wilkinson's catalyst; hydrogen / tetrahydrofuran / 16 h / 20 °C / 3040.2 Torr View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Iodine monochloride / dichloromethane; acetic acid / 5 h / 20 °C 2: copper(l) iodide; bis-triphenylphosphine-palladium(II) chloride; triethylamine / N,N-dimethyl-formamide / 3 h / 20 °C / Inert atmosphere 3: Wilkinson's catalyst; hydrogen / tetrahydrofuran / 16 h / 20 °C / 3040.2 Torr 4: formic acid / 3 h / 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol / 65 °C 2: potassium carbonate / N,N-dimethyl-formamide / 24 h / 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / methanol / 65 °C 2: potassium carbonate / N,N-dimethyl-formamide / 24 h / 20 °C / Inert atmosphere 3: formic acid / 3 h / 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Iodine monochloride / dichloromethane; acetic acid / 5 h / 20 °C 2: copper(l) iodide; bis-triphenylphosphine-palladium(II) chloride; triethylamine / N,N-dimethyl-formamide / 3 h / 20 °C / Inert atmosphere 3: Wilkinson's catalyst; hydrogen / tetrahydrofuran / 16 h / 20 °C / 3040.2 Torr 4: formic acid / 3 h / 20 °C / Inert atmosphere 5: triethylamine / acetonitrile / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydroxide / methanol / 65 °C 2: potassium carbonate / N,N-dimethyl-formamide / 24 h / 20 °C / Inert atmosphere 3: formic acid / 3 h / 20 °C / Inert atmosphere 4: triethylamine / acetonitrile / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / methanol / 3 h / Reflux 2: dicyclohexyl-carbodiimide; dmap / dichloromethane / 12 h / 20 °C / Reflux View Scheme |
-
-
143390-89-0
methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl)phenyl]acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide; water / methanol / 3 h / Reflux 2: dicyclohexyl-carbodiimide; dmap / dichloromethane / 12 h / 20 °C / Reflux 3: potassium iodide / acetonitrile / 24 h / Reflux View Scheme |
Kresoxim-methyl Chemical Properties
Molecular Structure of Kresoxim-methyl (CAS NO.143390-89-0):
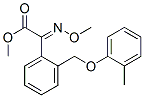
IUPAC Name: Methyl (2E)-2-methoxyimino-2-[2-[(2-methylphenoxy)methyl]phenyl]acetate
Molecular Formula: C18H19NO4
Molecular Weight: 313.35 g/mol
Density: 1.1 g/cm3
Melting Point: 98-100 °C
Boiling Point: 429.4 °C at 760 mmHg
Flash Point: 171.2 °C
Water Solubility: 2 mg/L (20 °C )
Storage Temp: 0-6 °C
Index of Refraction: 1.53
Molar Refractivity: 87.92 cm3
Molar Volume: 284.1 cm3
Surface Tension: 36.8 dyne/cm
XLogP3-AA: 4.1
H-Bond Acceptor: 5
Rotatable Bond Count: 7
Exact Mass: 313.131408
MonoIsotopic Mass: 313.131408
Topological Polar Surface Area: 57.1
Heavy Atom Count: 23
Canonical SMILES: CC1=CC=CC=C1OCC2=CC=CC=C2C(=NOC)C(=O)OC
Isomeric SMILES: CC1=CC=CC=C1OCC2=CC=CC=C2/C(=N\OC)/C(=O)OC
InChI: InChI=1S/C18H19NO4/c1-13-8-4-7-11-16(13)23-12-14-9-5-6-10-15(14)17(19-22-3)18(20)21-2/h4-11H,12H2,1-3H3/b19-17+
InChIKey: ZOTBXTZVPHCKPN-HTXNQAPBSA-N
Product Categories of Kresoxim-methyl (CAS NO.143390-89-0): Agricultural chemicals(bactericide)
Kresoxim-methyl Toxicity Data With Reference
| 1. | orl-rat LD50:5000 mg/kg | FEREAC Federal Register. 64 (1999),31130. | ||
| 2. | skn-rat LD50:2000 mg/kg | FEREAC Federal Register. 64 (1999),31130. | ||
| 3. | ihl-rat LC50:5.6 g/m3 | FEREAC Federal Register. 64 (1999),31130. |
Kresoxim-methyl Safety Profile
Hazard Codes:  Xn,
Xn,  N
N
Risk Statements: 40-50/53
R40:Limited evidence of a carcinogenic effect.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 2-36/37-60-61
S2:Keep out of the reach of children.
S36/37:Wear suitable protective clothing and gloves.
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN3077 9/PG 3
Moderately toxic by skin contact. Low toxicity by ingestion and inhalation. Questionable carcinogen with experimental data reported. When heated to decomposition it emits toxic vapors of NOx.
Kresoxim-methyl Specification
Kresoxim-methyl (CAS NO.143390-89-0) is also named as Bas 490F ; HSDB 7020 ; Methyl (E)-alpha-(methoxyimino)-2-((2-methylphenoxy)methyl)benzeneacetate ; Benzeneacetic acid, alpha-(methoxyimino)-2-((2-methylphenoxy)methyl)-, methyl ester, (E)- .
Related Products
- Kresoxim-methyl
- 143394-93-8
- 14340-01-3
- 14341-47-0
- 143415-62-7
- 14341-75-4
- 143417-80-5
- 14341-79-8
- 143418-49-9
- 14341-88-9
- 143426-48-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View