-
Name
Telatinib
- EINECS
- CAS No. 332012-40-5
- Article Data4
- CAS DataBase
- Density 1.418 g/cm3
- Solubility
- Melting Point
- Formula C20H16ClN5O3
- Boiling Point 713.595 °C at 760 mmHg
- Molecular Weight 409.832
- Flash Point 385.368 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Bay57-9352;Telatinib [INN];4-[[4-(4-Chloroanilino)furo[2,3-d]pyridazin-7-yl]oxymethyl]-N-methylpyridine-2-carboxamide;BAY-579352;2-Pyridinecarboxamide,4-[[[4-[(4-chlorophenyl)amino]furo[2,3-d]pyridazin-7-yl]oxy]methyl]-N-methyl-;UNII-18P7197Q7J;
- PSA 102.17000
- LogP 4.41730
Synthetic route
-
-
332013-43-1
4-(hydroxymethyl)-N-methylpyridine-2-carboxamide

-
-
332013-40-8
4-chloroanilino-7-chlorofuro[2,3-d]pyridazine

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether In toluene at 83 - 87℃; Product distribution / selectivity; | 46% |
| With potassium hydroxide; 18-crown-6 ether In toluene at 20 - 85℃; | 46% |
-
-
332013-43-1
4-(hydroxymethyl)-N-methylpyridine-2-carboxamide

-
-
332013-41-9
4-chloro-7-[N-(4-chlorophenyl)amino]-[2,3-d]furopyridazine

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether In toluene at 20 - 87℃; Product distribution / selectivity; | 46% |
| Stage #1: 4-(hydroxymethyl)-N-methylpyridine-2-carboxamide; 4-chloro-7-[N-(4-chlorophenyl)amino]-[2,3-d]furopyridazine; 18-crown-6 ether In toluene at 20℃; for 0.166667h; Stage #2: With potassium hydroxide In toluene at 80℃; for 24h; Product distribution / selectivity; |
-
-
332013-40-8
4-chloroanilino-7-chlorofuro[2,3-d]pyridazine

-
-
1182334-19-5
2-methylaminocarbonyl-4-pyridylcarbinol hydrochloride

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; toluene at 95℃; for 24h; Temperature; Time; Autoclave; | 37.7% |
-
-
123-39-7
N-Methylformamide

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| With sulfuric acid; hydroxylamine-O-sulfonic acid; iron(II) sulfate In water at 20℃; for 1.83333h; Product distribution / selectivity; | A 15.2% B n/a |
| Stage #1: N-Methylformamide; N-(4-chlorophenyl)-7-(4-pyridinylmethoxy)-furo[2,3-d]pyridazin-4-amine With sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid In water at 20℃; for 1.83333h; Stage #2: With sodium citrate In water at 0℃; for 0.166667h; Product distribution / selectivity; | A 15.2% B n/a |
| With sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid In water at 20℃; for 1.66667h; | A 15.2% B n/a |

-
-
332013-42-0
ethyl 2-methylaminocarbonyl-4-picolinate

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium tetrahydroborate / isopropyl alcohol; methanol / 8 h / 25 °C / Large scale 1.2: 1 h / 0 °C / Large scale 2.1: potassium tert-butylate / toluene; tetrahydrofuran / 24 h / 95 °C / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium tetrahydroborate / ethanol / 18 h / 20 °C 1.3: pH 9 2.1: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / ethanol / 18 h / 20 °C 2: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
13177-70-3
4,7-dichlorofuro[2,3-d]pyridazine

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol / 4 h / Heating / reflux 2: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 2 steps 1: ethanol / 4 h / Heating / reflux 2: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
13177-70-3
4,7-dichlorofuro[2,3-d]pyridazine

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: ethanol / 4 h / Heating / reflux 2.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 3.1: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.83 h / 20 °C 3.2: 0.17 h / 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1: ethanol / 4 h / Heating / reflux 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 3: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
1570-45-2
isonicotinic acid ethylester

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sulfuric acid; dihydrogen peroxide; iron(II) sulfate / water / 0.5 h / 6 - 22 °C 1.2: pH ~ 5 2.1: sodium tetrahydroborate / ethanol / 18 h / 20 °C 2.3: pH 9 3.1: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 3 steps 1: sulfuric acid; dihydrogen peroxide; iron(II) sulfate / water / 0.5 h / 6 - 22 °C 2: sodium tetrahydroborate / ethanol / 18 h / 20 °C 3: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
4282-24-0
furan-2,3-dicarboxylic acid

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: chloro-trimethyl-silane / 15.5 h / 20 °C 2.1: ethanol; water; hydrazine / 5.5 h / Heating / reflux 2.2: 4 h / Heating / reflux 3.1: pyridine; trichlorophosphate / 4 h / Heating / reflux 4.1: ethanol / 4 h / Heating / reflux 5.1: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 5 steps 1: chloro-trimethyl-silane / 15.5 h / 20 °C 2: hydrazine / ethanol / 5.5 h / Heating / reflux 3: pyridine; trichlorophosphate / 4 h / Heating / reflux 4: ethanol / 4 h / Heating / reflux 5: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
4282-24-0
furan-2,3-dicarboxylic acid

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: chloro-trimethyl-silane / 15.5 h / 20 °C 2.1: ethanol; water; hydrazine / 5.5 h / Heating / reflux 2.2: 4 h / Heating / reflux 3.1: pyridine; trichlorophosphate / 4 h / Heating / reflux 4.1: ethanol / 4 h / Heating / reflux 5.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 6.1: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.83 h / 20 °C 6.2: 0.17 h / 0 °C View Scheme | |
| Multi-step reaction with 6 steps 1: chloro-trimethyl-silane / 15.5 h / 20 °C 2: hydrazine / ethanol / 5.5 h / Heating / reflux 3: pyridine; trichlorophosphate / 4 h / Heating / reflux 4: ethanol / 4 h / Heating / reflux 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 6: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
332013-40-8
4-chloroanilino-7-chlorofuro[2,3-d]pyridazine

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 2.1: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.83 h / 20 °C 2.2: 0.17 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 2: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
52900-79-5
methyl 3-methoxycarbonylfuran-2-carboxylate

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: ethanol; water; hydrazine / 5.5 h / Heating / reflux 1.2: 4 h / Heating / reflux 2.1: pyridine; trichlorophosphate / 4 h / Heating / reflux 3.1: ethanol / 4 h / Heating / reflux 4.1: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrazine / ethanol / 5.5 h / Heating / reflux 2: pyridine; trichlorophosphate / 4 h / Heating / reflux 3: ethanol / 4 h / Heating / reflux 4: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
52900-79-5
methyl 3-methoxycarbonylfuran-2-carboxylate

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: ethanol; water; hydrazine / 5.5 h / Heating / reflux 1.2: 4 h / Heating / reflux 2.1: pyridine; trichlorophosphate / 4 h / Heating / reflux 3.1: ethanol / 4 h / Heating / reflux 4.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 5.1: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.83 h / 20 °C 5.2: 0.17 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1: hydrazine / ethanol / 5.5 h / Heating / reflux 2: pyridine; trichlorophosphate / 4 h / Heating / reflux 3: ethanol / 4 h / Heating / reflux 4: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 5: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
13177-71-4
5,6-dihydrofuro[2,3-d]pyridazine-4,7-dione

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine; trichlorophosphate / 4 h / Heating / reflux 2: ethanol / 4 h / Heating / reflux 3: potassium hydroxide; 18-crown-6 ether / toluene / 83 - 87 °C View Scheme | |
| Multi-step reaction with 3 steps 1: pyridine; trichlorophosphate / 4 h / Heating / reflux 2: ethanol / 4 h / Heating / reflux 3: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
13177-71-4
5,6-dihydrofuro[2,3-d]pyridazine-4,7-dione

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine; trichlorophosphate / 4 h / Heating / reflux 2.1: ethanol / 4 h / Heating / reflux 3.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 4.1: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.83 h / 20 °C 4.2: 0.17 h / 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1: pyridine; trichlorophosphate / 4 h / Heating / reflux 2: ethanol / 4 h / Heating / reflux 3: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 4: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
488-93-7
3-Furoic acid

-
-
332012-40-5
Telatinib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: n-butyllithium / tetrahydrofuran; hexanes / 2.5 h / -78 - -10 °C 2: chloro-trimethyl-silane / 15.5 h / 20 °C 3: hydrazine / ethanol / 5.5 h / Heating / reflux 4: pyridine; trichlorophosphate / 4 h / Heating / reflux 5: ethanol / 4 h / Heating / reflux 6: potassium hydroxide; 18-crown-6 ether / toluene / 20 - 85 °C View Scheme |
-
-
488-93-7
3-Furoic acid

-
A
-
332012-40-5
Telatinib

-
B
-
332012-41-6
4-(4-chlorophenylamino)-2-methylaminocarbonyl-7-(2-methylaminocarbonyl-4-pyridylmethoxy)-furo[2,3-d]pyridazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: n-butyllithium / tetrahydrofuran; hexanes / 2.5 h / -78 - -10 °C 2: chloro-trimethyl-silane / 15.5 h / 20 °C 3: hydrazine / ethanol / 5.5 h / Heating / reflux 4: pyridine; trichlorophosphate / 4 h / Heating / reflux 5: ethanol / 4 h / Heating / reflux 6: 1,8-diazabicyclo[5.4.0]undec-7-ene / 24 h / 125 °C 7: sulfuric acid; iron(II) sulfate; hydroxylamine-O-sulfonic acid / water / 1.67 h / 20 °C View Scheme |
-
-
332012-40-5
Telatinib

-
-
75-75-2
methanesulfonic acid

-
-
332013-26-0
4-[({4-[(4-chlorophenyl)amino]furan[2,3-d]pyridazin-7-yl}oxy)methyl]-N-methyl-2-pyridinecarboxamide methanesulfonate

| Conditions | Yield |
|---|---|
| In methanol for 0.25h; Autoclave; Reflux; Large scale; | 77.4% |
-
-
332012-40-5
Telatinib

-
-
104-15-4
toluene-4-sulfonic acid

-
-
332013-24-8
4-[({4-[(4-chlorophenyl)amino]furo[2,3-d]pyridazin-7-yl}oxy)methyl]-N-methyl-2-pyridinecarboxamide 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether for 0.166667h; | |
| Stage #1: Telatinib; toluene-4-sulfonic acid In methanol for 0.0833333h; Stage #2: In methanol; diethyl ether at 0℃; for 0.833333h; | |
| In methanol; diethyl ether at 0℃; for 0.916667h; |
Telatinib Chemical Properties
IUPAC Name: [(8Z,12Z,14E)-6-Hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-21-(prop-2-enylamino)-17-azabicyclo[16.3.1]docosa-1(21),8,12, 14,18-pentaen-10-yl] carbamate
Molecular Formula: C31H43N3O8
Molecular Weight: 585.69g/mol
Density: 1.21
Melting Point: 201-203°C
The Cas Register Number of Telatinib is 75747-14-7 .The chemical synonyms of Telatinib (CAS NO. 75747-14-7) are Geldanamycin,17-demethoxy-17-(2-propenylamino)- ; CP 127374 ; Allylaminogeldanamycin ; 17-(Allylamino)-17-demethoxygeldanamycin ; 17-(Allylamino)-17-desmethoxygeldanamycin ; 17-(Allylamino)-17-dimethoxygeldanamycin ; 17-Allylaminogeldanamycin ; 17-AG .The molecular structure of Telatinib (CAS NO. 75747-14-7) is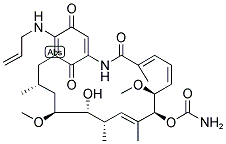 .
.
Telatinib Uses
It is a substance that is being studied in the treatment of cancer, specific young patients with certain types of leukemia or solid tumors, especially kidney tumors.
Telatinib Safety Profile
Hazard Codes: Xi
Xi
Risk Statements: 36/37/38
R36: Irritating to eyes.
R37: Irritating to respiratory system
R38: Irritating to skin.
Safety Statements: 22-24/25-36-26
S22: Do not breathe dust.
S24/25: Avoid contact with skin and eyes.
S36: Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
Telatinib Specification
The extinguishing agent of Telatinib (CAS NO. 75747-14-7) are dry powder, foam, sand, carbon dioxide, water mist.
Related Products
- Telatinib
- Telatinib
- 332013-42-0
- 332013-43-1
- 33203-82-6
- 33204-76-1
- 332052-58-1
- 33205-70-8
- 332062-01-8
- 332062-03-0
- 332062-06-3
- 332062-08-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View