-
Name
WITHAFERIN A
- EINECS
- CAS No. 5119-48-2
- Article Data4
- CAS DataBase
- Density 1.28
- Solubility
- Melting Point 252-253℃
- Formula C28H38 O6
- Boiling Point 680.7°C at 760 mmHg
- Molecular Weight 470.606
- Flash Point 226.7°C
- Transport Information
- Appearance
- Safety A poison by intraperitoneal route. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5b-Ergosta-2,24-dien-26-oic acid,5,6b-epoxy-4b,22,27-trihydroxy-1-oxo-, d-lactone, (20S,22R)- (8CI);Withaferin A (7CI); 5,6-Epoxy-5H-cyclopenta[a]phenanthrene,ergosta-2,24-dien-26-oic acid deriv.; NSC 101088; NSC 273757; Withaferine;Withaferine A
- PSA 96.36000
- LogP 3.35290
Synthetic route
-
-
1159096-16-8
2,3-dihydro-3β-O-sulfate withaferin A

-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With pyridine; potassium carbonate at 90℃; for 1h; | 93% |
| With carbon dioxide In dimethyl sulfoxide at 37℃; |

| Conditions | Yield |
|---|---|
| With Acnistus breviflorus In water for 72h; | A 4.0 mg B 5.6 mg |

| Conditions | Yield |
|---|---|
| With aluminum oxide In dichloromethane at 20℃; for 20h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 24h; | 99% |
-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; triethylamine In ethanol for 1h; | 98% |
-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 8h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.75h; Inert atmosphere; | 96% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
5119-48-2
withaferin-A

-
-
1392820-18-6
withaferin A 27-tert-butyldimethylsilyl ether

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In dichloromethane at 20℃; for 3h; | 94% |
| With 4-PP In N,N-dimethyl-formamide at 60℃; for 3h; | 90% |
| With 1H-imidazole; dmap In dichloromethane at 20℃; for 2.5h; | 89% |
| With dmap; triethylamine In dichloromethane at 20℃; for 12h; Reagent/catalyst; | |
| With dmap; triethylamine In dichloromethane at 20℃; for 0.8h; | 5.6 g |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 0.5h; | A 93% B 5% |

-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With iodine; triphenylphosphine In dichloromethane at 0 - 25℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran at 0 - 20℃; for 0.75h; Inert atmosphere; | A 3% B 91% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 85% |
-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With cerium(III) chloride heptahydrate In tetrahydrofuran; acetonitrile for 18h; Inert atmosphere; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 84% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 83% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 40h; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 78% |
-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With ammonium thiocyanate; 1,3,5-trichloro-2,4,6-triazine In tetrahydrofuran for 15h; Reflux; | 76% |
-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With iodine In chloroform at 20℃; for 1.5h; Cooling with ice; | 74% |
-
-
4648-54-8
trimethylsilylazide

-
-
5119-48-2
withaferin-A

-
-
1325214-13-8
2,3-dihydro,3-β-azido withaferin-A

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; pH=8.5; Michael-type addition; stereoselective reaction; | 73% |
-
-
108-24-7
acetic anhydride

-
-
5119-48-2
withaferin-A

-
A
-
1214886-35-7
27-acetoxy-5β,6β-epoxy-4β-hydroxy-1-oxo-witha-2,24-dienolide

-
B
-
22848-79-9
4,27-di-O-acetylwithaferin A

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 2.5h; | A 73% B 12% |
| With pyridine at 25℃; for 2h; | A 19% B 72% |

-
-
10328-92-4
N-Methylisatoic anhydride

-
-
5119-48-2
withaferin-A

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 24h; | 73% |
-
-
108-24-7
acetic anhydride

-
-
5119-48-2
withaferin-A

-
-
1214886-35-7
27-acetoxy-5β,6β-epoxy-4β-hydroxy-1-oxo-witha-2,24-dienolide

| Conditions | Yield |
|---|---|
| With pyridine at 25℃; for 2h; | 72% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydroxide In methanol; dichloromethane at 20℃; for 0.0833333h; | A 69% B 27% |

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 48h; | 66% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In dichloromethane at 20℃; for 20h; Inert atmosphere; | 66% |
Withaferine A Chemical Properties
Molecular structure of Withaferin A (CAS NO.5119-48-2) is:
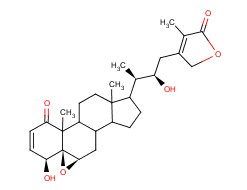
Product Name: Withaferin A
CAS Registry Number: 5119-48-2
Molecular Weight: 470.59772 [g/mol]
Molecular Formula: C28H38O6
XLogP3-AA: 3.8
H-Bond Donor: 2
H-Bond Acceptor: 6
Surface Tension: 55.1 dyne/cm
Density: 1.28 g/cm3
Flash Point: 226.7 °C
Enthalpy of Vaporization: 114.26 kJ/mol
Boiling Point: 680.7 °C at 760 mmHg
Vapour Pressure: 1.85E-21 mmHg at 25°C
Withaferine A Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 54mg/kg (54mg/kg) | Toxicon. Vol. 8, Pg. 154, 1970. |
Withaferine A Safety Profile
A poison by intraperitoneal route. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
Hazard Codes: T
T
Withaferine A Specification
Withaferin A , its cas register number is 5119-48-2. It also can be called 5-19-06-00604 (Beilstein Handbook Reference) ; BRN 1335150 ; NSC 273757 ; NSC-101088 ; WITHAFERIN DERIV JPR, IOWA U. COMPOUND ; Withaferine A ; (4beta,5beta,6beta,22R)-5,6-Epoxy-4,22,27-trihydroxy-1-oxoergosta-2,24-dien-26-oic acid, delta-lactone ; 5-beta-Ergosta-2,24-dien-26-oic acid, 5,6-beta-epoxy-4-beta,22,27-trihydroxy-1-oxo-, delta-lactone, (20S,22R)- ; 5beta-Ergosta-2,24-dien-26-oic acid, 5,6beta-epoxy-4beta,22,27-trihydroxy-1-oxo-, delta-lactone, (20S,22R)-(8CI) ; Ergosta-2,24-dien-26-oic acid, 5,6-epoxy-4,22,27-trihydroxy-1-oxo-, gamma-lactone, (4bta,5beta,6beta,22R)- . Withaferin A (CAS NO.5119-48-2) is should be isolated from leaves of Withania somnifera Dun.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View