-
Name
YTTRIUM CHLORIDE
- EINECS 233-801-0
- CAS No. 10361-92-9
- Article Data75
- CAS DataBase
- Density 2.67 g/mL at 25 °C(lit.)
- Solubility Soluble.
- Melting Point 721 °C(lit.)
- Formula Cl3Y
- Boiling Point °Cat760mmHg
- Molecular Weight 195.265
- Flash Point °C
- Transport Information
- Appearance powder
- Safety Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of Cl−. See also YTTRIUM and RARE EARTHS.
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Yttriumchloride;Yttrium chloride (Y2Cl6);Yttrium trichloride;Yttrium(III) chloride;
- PSA 0.00000
- LogP 2.06850
Synthetic route

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) byproducts: H2O; Schlenk techniques; heating Y compd. under vacuum (1E-3 Torr); | 99% |

| Conditions | Yield |
|---|---|
| With pyrographite In gas byproducts: CO; by chlorination-chem. vapor transport react.; mixt. of Er2O3 and Y2O3 with carbon and KCl (1:1:6:1 at. ratio) placed in alumina reactor and chlorinated with dry Cl2 (20 cm**3/min) at 800 K for 2 h; gas replace by Ar:Cl2 at 800-1300 K; detn. of separation factor; | A 31.74% B 21.33% |


| Conditions | Yield |
|---|---|
| In hydrogenchloride Y2O3 dissolved in 10 ml HCl (6m); addn. of solid NH4Cl (n(NH4Cl):n(Y2O3) approx. 15), addn. of 10 ml concd. HCl, heating to near dryness, cooling, ground; transferred to a quartz tube with a side arm, evacuation to ca. 10 Pa, heated to 373 K for 15-18 h, then temp. rise to 673 K (sublimation of NH4Cl), then temp. rise to 1223 K (sublimation of YCl3); elem. anal.; |


| Conditions | Yield |
|---|---|
| Y2O3 is reacted with NH4Cl; purified by two distn. in tantalum crucibles under high vac.; | |
| In hydrogenchloride react. of oxide and excess NH4Cl in concd. HCl at 100°C; sublimation at 360°C/0.1 mmHg; | |
| In not given extd. with THF; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating (70°C, vac.; 100-250°C, HCl atm.); |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| With NH4Cl In neat (no solvent) heating of YCl3*99H2O in the presence of NH4Cl (Handbuch der preparativen anorganischen Chemie, Herausg. G. Brauer, Studgardt, 1975); |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) thermolysis (vac., 400°C); sublimation (870°C, 1E-5 torr); | |
| In neat (no solvent) Y compd. transferred to platinum crucible under dry conditions, evacuation temp. raised slowly to 350-400°C, complete decompn. after fewhs.; |

-
-
7782-50-5
chlorine

-
-
7429-90-5
aluminium

-
A
-
1333-84-2, 1344-28-1
aluminum oxide

-
B
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating (200°C, vac.; 300°C, 2 d); separated by chemical transport; |


| Conditions | Yield |
|---|---|
| In melt exchange reaction (KCl/NaCl melt, 1073 K); | |
| In melt exchange reaction (KCl-NaCl melt, 1073 K); |


| Conditions | Yield |
|---|---|
| at 300°C, 1-2 d, sealed tube; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) passing stream of COCl2 over Y2O3 at 600 °C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) vac., 573 K; vapour trasportion; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) in streaming Cl2-gas at 200-300°C;; |

| Conditions | Yield |
|---|---|
| at 500°C, 6 h; | |
| In neat (no solvent) chlorination of Y2O3 with carbon tetrachloride at 955 K for 10 h; elem. anal.; |

| Conditions | Yield |
|---|---|
| With Cl2 In neat (no solvent) 250-300°C, 1 d; Cl2 atm., 600°C; |

| Conditions | Yield |
|---|---|
| In water dissolving Y2O3 in HCl soln.; evapn.; | |
| In neat (no solvent) oxide was treated with concd. HCl; evapd.; residue dissolved in H2O; evapd.; | |
| In hydrogenchloride metal oxide dissolved in slight excess of HCl; solvent evapd.; |

-
B
-
10361-92-9
yttrium(III) chloride

-
C
-
7646-78-8
tin(IV) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) thermic decomposition;; |


| Conditions | Yield |
|---|---|
| With pyrographite In solid byproducts: CO; at 800°C for 2 h; | |
| With aluminium trichloride; pyrographite In neat (no solvent) chlorinating of rare earth/carbon (molar ration 3/1) mixt. (Cl2 flow rate 20 ml/min, 800 K, 2 h), heating in CO/HCl flow (800-1200 K), chemical vapor transport (AlCl3, 1300 K, 6 h, CO carrier gas, 40 ml/min); atomic emission spectrometric monitoring; | |
| With sucrose carbon In neat (no solvent) byproducts: CO, CO2, O2; mixt. of Y2O3 and sucrose carbon kept under flowing Ar for 1 h and heated at 100°C; Cl2 introduced; mixt. heated in Ar/Cl2 atm. up to 950°C at 3.8°C/min; X-ray diffraction; | |
| With sucrose carbon In further solvent(s) Kinetics; byproducts: CO, CO2; the chlorination kinetics of Y2O3/sucrose carbon studied by thermogravimetry at 550-950°C; Cl2 used in a mixt. with Ar; |

| Conditions | Yield |
|---|---|
| With chlorine byproducts: CO; at 1100°C; YCl3 removed from the reaction zone with a stream of He; | |
| With Cl2 byproducts: CO; at 1100°C; YCl3 removed from the reaction zone with a stream of He; |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| With ammonium chloride In neat (no solvent) heating of mixt. of YCl3-hydrate and NH4Cl in Cl2/HCl stream; |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) 300°C, 2 d;; | |
| In neat (no solvent) 300°C, 2 d;; |

| Conditions | Yield |
|---|---|
| With HCl In hydrogenchloride byproducts: O2, H2O; dissolution in excess concd. HCl; |


| Conditions | Yield |
|---|---|
| With HCl In hydrogenchloride byproducts: O2, H2O; dissolution in excess concd. HCl; |


| Conditions | Yield |
|---|---|
| With HCl In hydrogenchloride byproducts: O2, H2O; dissolution in excess concd. HCl; |


| Conditions | Yield |
|---|---|
| With HCl In hydrogenchloride byproducts: O2, H2O; dissolution in excess concd. HCl; |


-
A
-
353-50-4
Carbonyl fluoride

-
B
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| byproducts: SO2; above 400°C; | |
| byproducts: SO2; above 400°C; |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| thermal decompn. at 500-1000°C; | |
| thermal decompn. at 500-1000°C; | |
| thermal decompn. at 500-1000°C; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) 335°C; thermogravimetric anal.; |

| Conditions | Yield |
|---|---|
| With HCl In hydrogenchloride byproducts: O2, H2O; dissolution in excess concd. HCl; |

-
-
101750-31-6
Y((SiMe3)2)2Cl(tetrahydrofuran)2

-
B
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| byproducts: THF; decompn. in absence of THF; |
-
-
6389-79-3
methyl methylphenylphosphinate

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: MeCl; slow heating in neat Ph(Me)P(O)OMe until complete pptn.; particle size and polymerization degree depending on heating rate; | 100% |
| In not given byproducts: chloromerhane; heating with the excess of methylphenylphosphinic acid methyl ester; filtering, washing with ethanol; |

| Conditions | Yield |
|---|---|
| In water stoich., YCl3 and 1,4-benzenedicarboxylate disodium salt mixed in H2O atroom temp.; filtered, dried (air), elem. anal.; | 100% |
| In water stoich. amt. of YCl3 and Na salt of terephthalic acid mixed in H2O; filtered; ppt. dried in air; detd. by X-ray powder diffraction and inductively coupled plasma-atomic emission spectra; |

-
-
10138-41-7
erbium(III) chloride

-
-
7732-18-5
water

-
-
10361-92-9
yttrium(III) chloride

-
-
10028-70-3
disodium terephthalate

| Conditions | Yield |
|---|---|
| In water stoich., ErCl3, YCl3 and 1,4-benzenedicarboxylate disodium salt mixed inH2O at room temp.; filtered, dried (air), elem. anal.; | 100% |

-
-
10138-41-7
erbium(III) chloride

-
-
7732-18-5
water

-
-
10361-92-9
yttrium(III) chloride

-
-
10028-70-3
disodium terephthalate

| Conditions | Yield |
|---|---|
| In water stoich., ErCl3, YCl3 and 1,4-benzenedicarboxylate disodium salt mixed inH2O at room temp.; filtered, dried (air), elem. anal.; | 100% |

-
-
10138-41-7
erbium(III) chloride

-
-
7732-18-5
water

-
-
10361-92-9
yttrium(III) chloride

-
-
10028-70-3
disodium terephthalate

| Conditions | Yield |
|---|---|
| In water stoich., ErCl3, YCl3 and 1,4-benzenedicarboxylate disodium salt mixed inH2O at room temp.; filtered, dried (air), elem. anal.; | 100% |

-
-
10138-41-7
erbium(III) chloride

-
-
7732-18-5
water

-
-
10361-92-9
yttrium(III) chloride

-
-
10028-70-3
disodium terephthalate

| Conditions | Yield |
|---|---|
| In water stoich., ErCl3, YCl3 and 1,4-benzenedicarboxylate disodium salt mixed inH2O at room temp.; filtered, dried (air), elem. anal.; | 100% |


-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In isopropyl alcohol byproducts: isopropyl acetate; molar ratio chloride:isopropoxide=2:1, refluxing; solvent removal (reduced pressure), drying (28°C, 0.1 torr); elem. anal.; | 99.9% |

| Conditions | Yield |
|---|---|
| In water byproducts: CO2, NH4Cl, H2O; to stirred soln. of YCl3 (5 mmol) was added NH4HCO3 (15 mmol) at 25° C; the soln. was maintained at 25°C for 1 wk; the resulting ppt. was filtered, washed with H2O and air-dried; chem. anal.; | 99.24% |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In isopropyl alcohol byproducts: isopropyl acetate; molar ratio chloride:isopropoxide=1:2, refluxing; solvent removal (reduced pressure), drying (28°C, 0.1 torr); elem. anal.; | 99.2% |
-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| With lithium In neat (no solvent) reduction of YCl3 (distd. in vac. at 900-950°C), 16.56kg YCl3, 2.040 kg Li (15.5% excess);; | 99% |
| With calcium In neat (no solvent) YCl3 free from H2O and oxide-halogenides, 10% excess Ca, under Ar, start of react. at 1350-1400.degreee.C;; heating to 1550°C for 5min after end of react., purity 99.-+.0.5%;; | 90% |
| With sodium In neat (no solvent) distn. of Na into a vessel containing YCl3 at 800-850°C and 0.07 atm Ar-pressure, 5-7h at 800-850°C, pouring off NaCl-slag;; distn. at 850°C; contaminations in wt.-%: 0.012-0.090 O, 0.002-0.03 N, 0.01-0.03 C;; | 85% |

-
-
10361-92-9
yttrium(III) chloride

-
-
10026-12-7
niobium pentachloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) Ar, stoichiometric mixture sealed in a quartz tube, heated at 1023 K for4 d, the heating and cooling ramps were 20 and 10 K*h**-1, respectively; | 99% |


| Conditions | Yield |
|---|---|
| In not given hot soln.; | 99% |
| In not given hot soln.; | 99% |

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide byproducts: KCl; YCl3 and Pd-complex were stirred in DMF at room temp. over 5 ds; filtered, DMF was pumped, 24 h at room temp., dried under vac. for 12-14h; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With NaH In tetrahydrofuran byproducts: NaCl; inert atmosphere; treatment of 6 equiv. of ligand with slight excess ofNaH, filtration, addn. to suspn. of YCl3, refluxing for 24 h; solvent removal (vac.), dissoln. in MePh, filtration (Celite), concn., crystn. (-30°C); elem. anal.; | 99% |

-
-
52687-02-2
sodium 2-(N,N-dimethylaminomethyl)phenolate

-
-
10361-92-9
yttrium(III) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: NaCl; N2-atmosphere; dropwise addn. of 3 equiv. ligand to metal chloride (roomtemp.), stirring overnight; solvent removal (vac.), extn. into CH2Cl2, solvent removal (vac.), washing (pentane); elem. anal.; | 99% |
Yttrium(III) chloride Chemical Properties
IUPAC Name: Trichloroyttrium
The MF of Yttrium(III) chloride (CAS NO.10361-92-9) is Cl3Y.
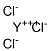
The MW of Yttrium(III) chloride (CAS NO.10361-92-9) is 195.26.
Synonyms of Yttrium(III) chloride (CAS NO.10361-92-9): Yttrium chloride ; Yttrium chloride (YCl3) ; Yttrium chloride solution ; Yttrium trichloride
Product Categories: Inorganics;Catalysis and Inorganic Chemistry;Chemical Synthesis;Crystal Grade Inorganics;Metal and Ceramic Science;Salts;Yttrium Salts
Density: 2.67 g/mL at 25 °C
Melting Point: 721 °C
Storage temp: 2-8 °C
Sensitive: Hygroscopic
EINECS: 1036-192-9
Merck: 14,10107
Form: powder
Yttrium(III) chloride Uses
Yttrium(III) chloride (CAS NO.10361-92-9) is used as analytical reagent.It is also used as a catalyst and in superconductors and has been linked to pulmonary edema and liver damage.
Yttrium(III) chloride Production
Yttrium(III) chloride can be formed by reacting yttrium(III) oxide with hydrochloric acid, among other methods.
Yttrium(III) chloride Toxicity Data With Reference
| 1. | ipr-rat LD50:45 mg/kg | AMIHAB AMA Archives of Industrial Health. 16 (1957),475. | ||
| 2. | ipr-mus LD50:88 mg/kg | AMIHAB AMA Archives of Industrial Health. 16 (1957),475. | ||
| 3. | ipr-gpg LD50:85 mg/kg | AEHLAU Archives of Environmental Health. 5 (1962),437. |
Yttrium(III) chloride Consensus Reports
Reported in EPA TSCA Inventory.
Yttrium(III) chloride Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of Cl−. See also YTTRIUM and RARE EARTHS.Safety information of Yttrium(III) chloride (CAS NO.10361-92-9):
Hazard Codes  Xi
Xi
Risk Statements
36/37/38 Irritating to eyes, respiratory system and skin
Safety Statements
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
37/39 Wear suitable gloves and eye/face protection
WGK Germany 3
RTECS ZG3150000
Yttrium(III) chloride Standards and Recommendations
ACGIH TLV: TWA 1 mg(Y)/m3
Yttrium(III) chloride Specification
Yttrium(III) chloride is an ionic compound of yttrium and chlorine. It is a salt that is solid at room temperature, highly soluble in water, and deliquescent. YCl3 in the solid state has a crystal structure with cubic close packed chloride ions and yttrium ions filling one third of the octahedral holes and the resulting YCl6 octahedra sharing three edges with adjacent octahedra give a layer structure. This structure is shared by a range of compounds notably AlCl3
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View