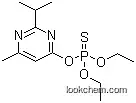
333-41-5 Usage
Description
Diazinon is a broad-spectrum, nonsystemic organophosphate insecticide and acetylcholinesterase inhibitor. It was formerly used in residential and agricultural settings to control various insect and pest species, functioning by inhibiting the enzyme acetylcholinesterase, which leads to an abnormal accumulation of the neurotransmitter acetylcholine and subsequent neural toxicity.
Used in Residential Pest Control:
Diazinon is used as an insecticide for controlling pests such as cockroaches, silverfish, ants, and fleas in residential and non-food buildings.
Used in Agricultural Pest Control:
Diazinon is used as an insecticide and cholinesterase inhibitor for controlling a wide range of sucking and chewing insects and mites in crops like soil, fruit, vegetables, and ornamentals.
Used in Veterinary Medicine:
Diazinon serves as a veterinary ectoparasiticide, used to control external parasites in animals.
Note: Diazinon's use has been restricted or discontinued in many regions due to its potential health risks and environmental concerns.
Air & Water Reactions
The neat compound is susceptible to oxidation and should be protected from prolonged exposure to air . Insoluble in water.
Health Hazard
LIQUID: POISONOUS IF SWALLOWED. Irritating to skin and eyes.
Health Hazard
Humans are exposed to diazinon during manufacture and professional applications. Diazinon
causes poisoning with symptoms such as headache, dizziness, nausea, weakness, feelings of
anxiety, vomiting, pupillary constriction, convulsions, respiratory distress or labored breathing,
unconsciousness, muscle cramp, excessive salivation, respiratory failure, and coma.
Health Hazard
Cholinesterase inhibitor; moderately toxic byingestion and skin absorption; ingestion ofapproximately large quantities of 10–15 gcan produce cholinergic effects in adulthuman; poisoning symptoms include headache, dizziness, weakness, blurred vision,pinpoint pupils, salivation, muscle spasms,vomiting abdominal pain, and respiratorydepression; LD50 values in experimentalanimals show wide variation.LD50 oral (rat): within the range 100–300mg/kgLD50 oral (guinea pig): 250 mg/kg.
Fire Hazard
Not flammable. POISONOUS GASES ARE PRODUCED WHEN HEATED. Oxides of sulfur and of phosphorus are generated in fires.
Trade name
AG-500?; AI3-19507?; ALFA-TOX?[C];
ANTIGAL?; ANTLAK?; BASUDIN?; BAZUDEN?;
CASWELL No. 342?; DACUTOX?; DASSITOX?;
DAZZEL?; DIAGRAN?; DIANON?; DIATERR-FOS?;
DIAZAJET?; DIAZATOL?; DIAZIDE?; DIAZINON AG
500 WBC?; DIAZINONE?; DIAZITOL?; DIAZOL?;
DICID?; DIMPYLATE?; DIPOFENE?; DIZIKTOL?;
DIZINON?[C]; DRAWIZON?; DYMET?; DYZOL?);
D.Z.N.?; EXODIN?; FEZUDIN?; FLYTROL?; G 301?;
G-24480?; GALESAN?; GARDENTOX?; GEIGY
24480?; KAYAZINON?; KAYAZOL?; NEOCIDOL?
(OIL); NEOCIDOL?; NIPSAN?; NUCIDOL?;
OLEODIAZINON?; ROOT GUARD; SAROLEX?[C];
SPECTRACIDE?; SROLEX?; SUZON?
Safety Profile
Poison by ingestion,
skin contact, subcutaneous, intravenous, and intraperitoneal routes. Mildly toxic by
inhalation. Human systemic effects by
ingestion: changes in motor activity, muscle
weakness, and sweating. Experimental
teratogenic and reproductive effects. A skin
and severe eye irritant. Human mutation
data reported. When heated to
decomposition it emits very toxic fumes of
NOx, POx, and SOx.
Potential Exposure
roducers, formulators and applicators
of this nonsystemic pesticide and acaricide. Diazinon is
used in the United States on a wide variety of agricultural
crops, ornamentals, domestic animals; lawns and gardens;
and household pests.
Carcinogenicity
Among 23,106 male applicators
participating in the Agricultural Health Study who
reported using diazinon, there was an increased risk with
exposure to diazinon for lung cancer, leukemia, and all
cancer sites combined, although the small number of cases
observed makes these estimates unreliable .
Environmental Fate
Biological. Sethunathan and Yoshida (1973a) isolated a Flavobacterium sp. (ATCC 27551) from rice paddy water that metabolized diazinon as the sole carbon source. Diazinon was readily hydrolyzed to 2-isopropyl-4-methyl-6-hydroxypyrimidine under aerobic conditions but less rapidly under anaerobic conditions. This bacterium as well as enrichment cultures isolated from a diazinon-treated rice field mineralized the hydrolysis product to carbon dioxide (Sethunathan and Pathak, 1971; Sethunathan and Yoshida, 1973). Rosenberg and Alexander (1979) demonstrated that two strains of Pseudomonas grew on diazinon and produced diethyl phosphorothioate as the major end product. The rate of microbial degradation increased in the presence of an enzyme (parathion hydrolase), produced by a mixed culture of Pseudomonas sp. (Honeycutt et al., 1984).
Soil. Hydrolyzes in soil to 2-isopropyl-4-methyl-2-hydroxypyrimidine, diethylphosphorothioic acid, carbon dioxide (Getzin, 1967; Lichtenstein et al., 1968; Sethunathan and Yoshida, 1969; Sethunathan and Pathak, 1972; Bartsch, 1974; Wolfe et al., 1976; Somasundaram and Coats, 1991) and tetraethylpyrophosphate (Paris and Lewis, 1973). The half-life of diazinon in soil was observed to be inversely proportional to temperature and soil moisture content (Getzin, 1968). Seven months after diazinon was applied on a sandy loam (2 kg/ha), only 1% of the total applied amount remained and 10% was detected in a peat loam (Suett, 1971).
The reported half-life in soil is 32 days (Jury et al., 1987). Reported half-lives in soil following incubation of 10 ppm diazinon in sterile sand loam, sterile organic soil, nonsterile sandy loam and nonsterile organic soil are 12.5, 6.5, <1 and 2 weeks, respectively (Miles et al., 1979). The reported half-life of diazinon in sterile soil at pH 4.7 was 43.8 days (Sethunathan and MacRae, 1969). Major metabolites identified were diethyl thiophosphoric acid, 2-isopropyl-4-methyl-6-hydroxypyrimidine and carbon dioxide (Konrad et al., 1967). When soil is sterilized, the persistence of diazinon increased more so than changes in soil moisture, soil type or rate of application (Bro-Rasmussen et al., 1968). The halflives for diazinon in flooded soil incubated in the laboratory ranged from 4 to 17 days with an average half-life of 10 days (Sethunathan and MacRae, 1969; Sethunathan and Yoshida, 1969; Laanio et al., 1972). The mineralization half-life for diazinon in soil was 5.1 years (Sethunathan and MacRae, 1969; Sethunathan and Yoshida, 1969).
The half-lives of diazinon in a sandy loam, clay loam and an organic amended soil under nonsterile conditions were 66–1,496, 49–1,121 and 14–194 days, respectively, while under sterile conditions the half-lives were 57–1,634, 46–1,550 and 14–226 days, respectively (Schoen and Winterlin, 1987).
In a silt loam and sandy loam, reported Rf values were 0.86 and 0.88, respectively (Sharma et al., 1986).
Surface Water. In estuarine water, the half-life of diazinon ranged from 8.2 to 10.2 days (Lacorte et al., 1995).
Groundwater. According to the U.S. EPA (1986) diazinon has a high potential to leach to groundwater.
Plant. Diazinon was rapidly absorbed by and translocated in rice plants. Metabolites identified in both rice plants and a paddy soil were 2-isopropyl-4-methyl-6-hydroxypyrimidine (hydrolysis product), 2-(1¢-hydroxy-1¢-methyl)ethyl-4-methyl-6-hydroxypyrimidine and other polar compounds (Laanio et al., 1972). Oxidizes in plants to diazoxon (Ralls et al., 1966; Laanio et al., 1972; Wolfe et al., 1976) although 2-isopropyl-4-methyl- 6-pyrimidin-6-ol was identified in bean plants (Kansouh and Hopkins, 1968) and as a hydrolysis product in soil (Somasundaram et al., 1991) and water (Suffet et al., 1967). Five days after spraying, pyrimidine ring-labeled 14C-diazinon was oxidized to oxodiazinon which was then hydrolyzed to 2-isopropyl-4-methylpyrimidin-6-ol which in turn, was further metabolized to carbon dioxide (Ralls et al., 1966). Diazinon was transformed in field-sprayed kale plants to form hydroxydiazinon {O,O-diethyl-O-[2-(2¢-hydroxy-2¢-propyl)- 4-methyl-6-pyrimidinyl] phosphorothioate} which was not previously reported (Pardue et al., 1970).
Metabolic pathway
The main route of diazinon metabolism in soil, plants and animals is
through cleavage of the P-O-pyrimidine group to yield 2-isopropyl-4-
methyl-6-hydroxypyrimidine. As with most other phosphorothioates,
loss of the pyrimidinyl function in mammalian metabolism probably
occurs either through oxidative desulfuration of the thiono group, catalysed
by microsomal mixed function oxidases, to give diazoxon followed
by hydrolysis catalysed by an A-esterase, or via an oxidative mechanism
catalysed by a mixed function oxidase acting directly on diazinon. In the
first case the second product is diethyl phosphate and in the second,
diethyl phosphorothioate (Yang et al., 1971). Further metabolism then
leads to hydroxylation of the methyl and isopropyl groups on the pyrimidine
ring. This oxidative metabolism may the act on the pyrimidinol,
diazoxon or diazinon itself, the last of which seems to be important in
mammalian and avian liver and gives rise to metabolites which still have
anticholinesterase or latent anticholinesterase activity.
Metabolism
The main biodegradation pathway in mammals, plants,
and soils is pyrimidinyl ester bond cleavage; the principal
metabolites are diethyl phosphorothioate and diethyl
phosphate. Degradation in the environment involves
oxidation to diazoxon and hydrolysis.
Shipping
UN2783 Organophosphorus pesticides, solid,
toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Toxicity evaluation
The acute oral LD50 for rats and mice are 1250
and 80–135 mg/kg. Inhalation LC50 (4 h) for rats is
>2330 mg/m3 air. NOEL (2 yr) for rats is 0.06 mg/kg/d.
Degradation
Diazinon(1) is stable at neutral pH but is slowly hydrolysed in alkaline
solutions and rapidly at acidic pH values (PM). Hydrolysis products
were identified as the pyrimidinol (2) and diethyl phosphorothioate (3)
(PSD, 1991).
When diazinon was dissolved in a water/soil suspension and irradiated
with UV light, Mansour et al. (1997) demonstrated that one of the
products was iso-diazinon (4) formed via a thiono-thiolo rearrangement
(see Scheme 1).
Incompatibilities
Reaction with nitrosating agents (e.g.,
nitrites, nitrous gases, nitrous acid) capable of releasing carci-
nogenic nitrosamines. Hydrolyzes slowly in water and dilute
acid. Reacts with strong acids and alkalis with possible forma-
tion of highly toxic tetraethyl thiopyrophosphates.
Incompatible with copper-containing compounds. Contact
with oxidizers may cause the release of phosphorous oxides.
Contact with strong reducing agents, such as hydrides; may
cause the formation of flammable and toxic phosphine gas.
Waste Disposal
Diazinon is hydrolyzed in
acid media about 12 times as rapidly as parathion, and
at about the same rate as parathion in alkaline media. In
excess water this compound yields diethylthiophosphoric
acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine. With
insufficient water, highly toxic tetraethyl monothiopyropho-
sphate is formed. Therefore, incineration would be a prefer-
able ultimate disposal method with caustic scrubbing of
the incinerator effluent
. In accordance with 40CFR165,
follow recommendations for the disposal of pesticides and
pesticide containers. Must be disposed properly by follow-
ing package label directions or by contacting your local or
federal environmental control agency, or by contacting
your regional EPA office.
Precautions
Workers should avoid eye contact with diazinon, wear chemical safety glasses or goggles,
protective clothing or equipment, wear waterproof boots, long-sleeved shirts, long pants,
and a hat. Workers should avoid contamination of food and feed, wash thoroughly after
handling and before eating or smoking. In fact, occupational workers should avoid eating,
drinking, or smoking in areas of work with the chemical.
References
Uner, N, et al. "Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus." Environmental Toxicology & Pharmacology 21.3(2006):241.
Shishido, Takashi, K. Usui, and J. I. Fukami. "Oxidative metabolism of diazinon by microsomes from rat liver and cockroach fat body." Pesticide Biochemistry & Physiology 2.1(1972):27-38.
Giordano, G, et al. "Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency." Toxicology & Applied Pharmacology 219.2-3(2007):181.
Check Digit Verification of cas no
The CAS Registry Mumber 333-41-5 includes 6 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 3 digits, 3,3 and 3 respectively; the second part has 2 digits, 4 and 1 respectively.
Calculate Digit Verification of CAS Registry Number 333-41:
(5*3)+(4*3)+(3*3)+(2*4)+(1*1)=45
45 % 10 = 5
So 333-41-5 is a valid CAS Registry Number.
InChI:InChI=1/C4H4N2O/c7-4-2-1-3-5-6-4/h1-3H,(H,6,7)
333-41-5Relevant articles and documents
Concerted rate-limiting proton transfer to sulfur with nucleophilic attack at phosphorus - A new proposed mechanism for hydrolytic decomposition of the P=S pesticide, Diazinon, in moderately acidic sulfuric acid media
Churchill, Doreen,Dust, Julian M.,Buncel, Erwin
, p. 421 - 431 (2007)
We report herein the first kinetic study of a P=S containing organophosphorus pesticide, Diazinon (1), in the moderately concentrated acid region. Product analyses (31P NMR) show that reaction occurs only at the P centre. The rate-acidity profile (kobs vs. molarity of H 2SO4) appears as a curve in which the initial slight downward trace (molarity = 1 to ca. 5) is followed by sharper upward curve (molarity ca. 5 to 14). Using treatments involving the excess acidity (X) method, the A-1 and A-2 mechanistic possibilities were found to be inoperative over the full acidity range. A novel mechanism is proposed for the higher acidity (X ca. 2-6) region. This mechanism involves proton transfer to P=S from hydronium ion with concomitant proton transfer from water, which effectively delivers hydroxide to the P centre in a variant of the A-SE2 process. A putative A-2 mechanism in this region is supplanted by the proposed A-S E2 variant where the cyclic array results in proton transfer being efficiently coupled with nucleophilic attack involving water. This constitutes the first report of rate-limiting proton transfer at the P=S functionality in acid hydrolysis of this class of organophosphorus neutroxins. A 600 000-fold acceleration in the decomposition of Diazinon is associated with the change of medium from neutral aqueous solution to the most acidic medium studied (X ca. 6).
-
Louloudes et al.
, p. 685 (1956)
-
Polymerization of Multifunctional Azides, and Polymers Therefrom
-
, (2011/04/18)
Methods for preparing polymers from multifunctional azides and multifunctional azide-reactants are described in the present disclosure. Exemplary multifunctional azide-reactants include multifunctional alkynes and/or multifunctional α-phosphine esters. In certain embodiments, such polymers can be prepared in vivo. Such polymers can be useful in a wide variety of biomedical applications.
Cis-Alkoxyspiro-Substituted Tetramic Acid Derivatives
-
, (2008/06/13)
The invention relates to novel cis-alkoxyspiro-substituted tetramic acid derivatives of the formula (I), in which A, G, X, Y and Z are as defined above, to a plurality of processes and intermediates for their preparation and to their use as pesticides and/or herbicides, and also to selective herbicidal compositions comprising firstly cis-alkoxyspiro-substituted tetramic acid derivatives and secondly a crop plant compatibility-improving compound.