-
Name
3-Amino-4-methylbenzoic acid
- EINECS 219-543-1
- CAS No. 2458-12-0
- Article Data13
- CAS DataBase
- Density 1.254 g/cm3
- Solubility Very soluble in water.
- Melting Point 164-168 °C(lit.)
- Formula C8H9NO2
- Boiling Point 363 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 173.3 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-amino-4-methyl-benzoate;3-Amino-p-toluic acid;4-Methyl-3-aminobenzoic acid;
- PSA 63.32000
- LogP 1.85660
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In methanol for 5h; | 48% |
| With hydrogenchloride; tin | |
| With hydrogenchloride; tin(ll) chloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 110℃; im Rohr; |
-
-
60710-80-7
5-cyano-2-methylaniline

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; im Druckrohr; |

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| With sodium amalgam |



| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: nitric acid / 12 - 15 °C 2: nitric acid 3: sodium amalgam View Scheme |
-
-
859816-42-5
1-bromo-2-isopropyl-5-methyl-4-nitro-benzene

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid 2: sodium amalgam View Scheme |
-
-
861525-09-9
acetic acid-(5-chloroacetyl-2-methyl-anilide)

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen peroxide; alkali 2: hydrochloric acid / 110 °C / im Rohr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: fuming nitric acid 2: tin dichloride; hydrochloric acid View Scheme |
-
-
90178-80-6
4-(chloromethyl)-3-nitrobenzonitrile

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tin; fuming hydrochloric acid 2: fuming hydrochloric acid / 100 °C / im Druckrohr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: concentrated sulfuric acid; KNO3 / Giessen in Eiswasser 2: tin; fuming hydrochloric acid 3: fuming hydrochloric acid / 100 °C / im Druckrohr View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2458-12-0
3-amino-p-toluic acid

-
-
231958-04-6
3-[(tert-butyloxycarbonyl)amino]-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 18h; | 100% |
| In tetrahydrofuran at 50℃; | 91% |
| In tetrahydrofuran at 50℃; | 88% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
745048-63-9
5-amino-2-bromo-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 5 - 15℃; for 1.08h; Inert atmosphere; | 100% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 0℃; for 1.16667h; Inert atmosphere; | 89% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; | 89% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
2592-95-2
benzotriazol-1-ol

-
-
765-30-0
Cyclopropylamine

-
-
623155-19-1
3-amino-N-cyclopropyl-4-methylbenzamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; DMF (N,N-dimethyl-formamide) at 54℃; for 1.83333h; | 100% |

-
-
2458-12-0
3-amino-p-toluic acid

-
-
765-30-0
Cyclopropylamine

-
-
623155-19-1
3-amino-N-cyclopropyl-4-methylbenzamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap In N,N-dimethyl-formamide at 0 - 20℃; | 100% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; | 100% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In DMF (N,N-dimethyl-formamide) at 20℃; | 72% |
| With dmap In water; ethyl acetate; N,N-dimethyl-formamide | 72% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
108-24-7
acetic anhydride

-
-
6946-14-1
3-acetylamino-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| In acetic acid Acetylation; | 98% |
| In acetic acid at 20℃; for 1h; | 96.2% |
| With acetic acid at 20℃; for 16h; | 90% |
| In acetic acid at 20℃; | 87.6% |

| Conditions | Yield |
|---|---|
| With thionyl chloride Heating; | 98% |
| With sulfuric acid for 12h; Reflux; Inert atmosphere; | 98% |
| With thionyl chloride | 98% |
| With sulfuric acid at 75℃; for 31h; | 90% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
75-66-1
2-methylpropan-2-thiol

-
-
478169-77-6
3-(tert-butylthio)diazenyl-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 1h; | 96% |
-
-
137-26-8
Thiram

-
-
2458-12-0
3-amino-p-toluic acid

-
-
242800-82-4
3-(N,N-dimethylthioureido)-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; thiocarbamoylation; | 95% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
2592-95-2
benzotriazol-1-ol

-
-
54884-19-4
3-amino-4-methylbenzoic acid methylamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; methylamine In tetrahydrofuran; DMF (N,N-dimethyl-formamide) at 54℃; for 2h; | 95% |
-
-
67-56-1
methanol

-
-
2458-12-0
3-amino-p-toluic acid

-
-
18595-18-1
3-amino-4-methyl benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride at 5℃; for 6h; Reflux; | 94.9% |
| With thionyl chloride at 5℃; for 6h; Reflux; | 94.9% |
| With thionyl chloride at 5℃; for 6h; Reflux; | 94.9% |
| With sulfuric acid Heating; | |
| With thionyl chloride at 0℃; for 1h; Reflux; |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
3682-12-0
diethyltin(IV) oxide

-
-
141368-92-5
diethyltin(IV) bis(3-amino-4-methylbenzoate)

| Conditions | Yield |
|---|---|
| In ethanol; toluene according to: M. Boualam et al., Appl. Organomet. Chem. 4 (1990) 335; addn. of diorganotin oxide to soln. (toluene/ethanol 4:1) of 3-amino-4-methylbenzoic acid (molar ratio 2:1), refluxing (4 h), azeotrop distn.; evapn. (red. pressure), recrystn. (chloroform/petroleum ether); | 93% |

| Conditions | Yield |
|---|---|
| In DMF (N,N-dimethyl-formamide) at 20℃; for 16h; | 92% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
532-55-8
Benzoyl isothiocyanate

-
-
461413-31-0
N-(benzoyl)-N'-(3-carboxy-6-methylphenyl)thiourea

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 92% |
-
-
427878-41-9
4-chloro-5-methyl-pyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid ethyl ester

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 16h; | 92% |
-
-
56442-17-2
4-((4-fluorobenzyl)oxy)benzaldehyde

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-((4-fluorobenzyl)oxy)benzaldehyde; 3-amino-p-toluic acid In toluene for 2h; Reflux; Dean-Stark; Stage #2: With sodium tris(acetoxy)borohydride; acetic acid In tetrahydrofuran at 25℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol; toluene according to: M. Boualam et al., Appl. Organomet. Chem. 4 (1990) 335; addn. of diorganotin oxide to soln. (toluene/ethanol 4:1) of 3-amino-4-methylbenzoic acid (molar ratio 1:1), refluxing (4 h), azeotrop distn.; evapn. (red. pressure), recrystn. (toluene/ethanol); | 91% |
-
-
75-15-0
carbon disulfide

-
-
2458-12-0
3-amino-p-toluic acid

-
-
114379-99-6
3-isothiocyanato-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; 3-amino-p-toluic acid With triethylamine In tetrahydrofuran; water at 20℃; for 26h; Stage #2: With iodine In tetrahydrofuran; water at 0℃; for 2.03333h; Stage #3: With hydrogenchloride In tetrahydrofuran; water | 91% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
818-08-6
di(n-butyl)tin oxide

-
-
141368-91-4
di-n-butyltin(IV) bis(3-amino-4-methylbenzoate)

| Conditions | Yield |
|---|---|
| In ethanol; toluene according to: M. Boualam et al., Appl. Organomet. Chem. 4 (1990) 335; addn. of diorganotin oxide to soln. (toluene/ethanol 4:1) of 3-amino-4-methylbenzoic acid (molar ratio 2:1), refluxing (4 h), azeotrop distn.; evapn. (red. pressure), recrystn. (chloroform/ethanol); | 89% |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
98555-09-0
3-azido-4-methyl-benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-amino-p-toluic acid With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5h; Acidic aqueous solution; Stage #2: With sodium azide In water at 0℃; Acidic aqueous solution; | 88% |
| With hydrogenchloride; sodium azide; sodium nitrite In water | 88% |
| Stage #1: 3-amino-p-toluic acid With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5h; Stage #2: With sodium azide In water for 0.5h; |

| Conditions | Yield |
|---|---|
| In DMF (N,N-dimethyl-formamide) at 60℃; for 3h; | 88% |
-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| With formic acid In water for 2h; Heating / reflux; | 88% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 60℃; for 3h; | 88% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 60 - 100℃; |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
78933-24-1
pyrazolo[1,5-a]pyridine-3-carbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.5h; | 87% |

| Conditions | Yield |
|---|---|
| With triethylamine; HATU In dichloromethane | 83% |
-
-
919084-99-4
6-(2-chloro-benzoimidazol-1-yl)-pyrimidin-4-ylamine

-
-
2458-12-0
3-amino-p-toluic acid

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In 1,3-dimethyl-2-imidazolinone at 80℃; for 12h; | 82% |

| Conditions | Yield |
|---|---|
| With Fe(SO3C6H4NO2)3*4.5H2O In sulfuric acid at 140℃; for 4h; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-amino-p-toluic acid With acetic anhydride; acetic acid at 60℃; for 0.75h; Stage #2: 3-amino-p-toluic acid With 3-acetylamino-4-methyl-2-nitrobenzoic acid; acetic anhydride for 0.5h; Heating / reflux; | 76% |

| Conditions | Yield |
|---|---|
| In ethanol; toluene according to: M. Boualam et al., Appl. Organomet. Chem. 4 (1990) 335; addn. of diorganotin oxide to soln. (toluene/ethanol 4:1) of 3-amino-4-methylbenzoic acid (molar ratio 1:1), refluxing (4 h), azeotrop distn.; evapn. (red. pressure), recrystn. (chloroform/ethanol); | 76% |
-
-
870221-17-3
4-(2-chloropyridin-3-yl)pyrimidine

-
-
2458-12-0
3-amino-p-toluic acid

-
-
870221-16-2
4-methyl-3-(3-(pyrimidin-4-yl)pyridin-2-ylamino)benzoic acid

| Conditions | Yield |
|---|---|
| With triethylamine triflouroacetate In dimethyl sulfoxide at 95℃; for 65h; | 71% |
| With triethylamine triflouroacetate In dimethyl sulfoxide at 95℃; for 65h; |
-
-
2458-12-0
3-amino-p-toluic acid

-
-
129694-52-6
ethyl (Z)-N-(2-amino-1,2-dicyanovinyl)formamidate

-
-
931424-02-1
3-{[(Z)-2-amino-1,2-dicyanovinyl]amino}methyleneamino-4-methylbenzoic acid

| Conditions | Yield |
|---|---|
| 1,8-diazabicyclo[5.4.0]undec-7-ene In methanol at 20℃; for 24h; | 70% |
3-Amino-4-methylbenzoic acid Chemical Properties
Product Name: 3-Amino-4-methylbenzoic acid (CAS NO.2458-12-0)
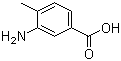
Molecular Formula: C8H9NO2
Molecular Weight: 151.16g/mol
Mol File: 2458-12-0.mol
EINECS: 219-543-1
Melting Point: 164-168 °C(lit.)
Boiling point: 363 °C at 760 mmHg
Flash Point: 173.3 °C
Density: 1.254 g/cm3
Index of Refraction: 1.618
Molar Refractivity: 42.24 cm3
Molar Volume: 120.5 cm3
Surface Tension: 58.2 dyne/cm
Enthalpy of Vaporization: 64.25 kJ/mol
Vapour Pressure: 6.64E-06 mmHg at 25°C
XLogP3-AA: 0.3
H-Bond Donor: 2
H-Bond Acceptor: 3
Structure Descriptors of 3-Amino-4-methylbenzoic acid (CAS NO.2458-12-0):
IUPAC Name: 3-amino-4-methylbenzoic acid
Canonical SMILES: CC1=C(C=C(C=C1)C(=O)O)N
InChI: InChI=1S/C8H9NO2/c1-5-2-3-6(8(10)11)4-7(5)9/h2-4H,9H2,1H3,(H,10,11)
InChIKey: XKFIFYROMAAUDL-UHFFFAOYSA-N
Product Categories: CARBOXYLICACID; Chemical intermediate for Nilotinib; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Benzoic acid; Organic acids
3-Amino-4-methylbenzoic acid Safety Profile
Safety Information about 3-Amino-4-methylbenzoic acid (CAS NO.2458-12-0):
Hazard Codes Xi 
Risk Statements 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements 26-36-24/25
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
FIRST AID MEASURES
Eyes:Flush eyes with plenty of water for at least 15 minutes,occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:If victim is conscious and alert, give 2-4 cupfuls of milk or water.Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult,give oxygen. Get medical aid.
3-Amino-4-methylbenzoic acid Specification
3-Amino-4-methylbenzoic acid , its CAS NO. is 2458-12-0, the synonym is 3-Amino-p-toluic acid .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View