-
Name
4'-Aminoacetanilide
- EINECS 204-576-6
- CAS No. 122-80-5
- Article Data137
- CAS DataBase
- Density 1.203 g/cm3
- Solubility 0.1-1 g/100 mL at 25 °C
- Melting Point 164-167 °C(lit.)
- Formula C8H10N2O
- Boiling Point 267.726 °C at 760 mmHg
- Molecular Weight 150.18
- Flash Point 115.717 °C
- Transport Information
- Appearance pink to brown fine crystalline powder
- Safety 22-26-36/37-39-36
- Risk Codes 36-42/43-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Acetanilide,4'-amino- (6CI,7CI,8CI);Acetanilide, p-amino- (4CI);(4-(Acetylamino)phenyl)amine;1-Amino-4-(acetylamino)benzene;4-(Acetylamino)aniline;4-Acetylaminobenzeneamine;Acetparamin;Acetyl-p-phenylenediamine;C.I. 76005;C.I.Oxidation Base 19;Fourrine 88;Fourrine A;N-(4-Aminophenyl)acetamide;N-(p-Aminophenyl)acetamide;N-Acetyl-1,4-benzenediamine;N-Acetyl-p-phenylenediamine;NSC 2135;Paracetamin;p-(Acetylamino)aniline;p-Acetamidoaniline;p-Aminoacetanilide;
- PSA 55.12000
- LogP 1.88140
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; nickel In methanol for 6h; | 100% |
| With indium; acetic acid In tetrahydrofuran Heating; | 100% |
| With trimethylamine-borane; palladium hydroxide - carbon In methanol for 3.5h; Heating; | 99% |
-
-
64-19-7
acetic acid

-
-
141-78-6
ethyl acetate

-
-
106-50-3
1,4-phenylenediamine

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane at 65 - 90℃; for 6h; Time; Inert atmosphere; | 98.6% |

-
-
38063-81-9
p-aminoacetophenone oxime

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In toluene at 90℃; for 0.5h; Beckmann rearrangement; | 98% |
| With trifluoromethylsulfonic anhydride In dichloromethane at 20℃; for 3h; Beckmann Rearrangement; Inert atmosphere; | 94% |
| With iodine In acetonitrile for 4h; Beckmann rearrangement; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| at 20℃; | 95% |
| In dichloromethane at 20℃; Inert atmosphere; | 95% |
| In tetrahydrofuran at 20℃; | 65% |

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; lithium hexamethyldisilazane In 1,4-dioxane at 90℃; for 2h; Glovebox; Sealed tube; | 91% |
| Stage #1: 4-bromoacetanilide With lithium hexamethyldisilazane; DavePhos; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane at 100℃; for 17h; Stage #2: With hydrogenchloride In 1,4-dioxane at 20℃; for 0.0833333h; Further stages.; | 70% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate; 1-(5,6,7,8-tetrahydroquinolin-8-yl)-2-methylpropan-1-one; copper(I) bromide In dimethyl sulfoxide at 25℃; for 24h; Inert atmosphere; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate In dimethyl sulfoxide at 80℃; UV-irradiation; | 89% |

-
-
106-50-3
1,4-phenylenediamine

-
A
-
16937-03-4
4-nitro-N-(4-chlorophenyl)benzenesulfonamide

-
B
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 80℃; for 2h; Green chemistry; chemoselective reaction; | A n/a B 86% |

-
-
52578-66-2
N-(4-azido-phenyl)acetamide

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With iron(III) oxide; hydrazine hydrate In water at 120℃; for 1.5h; Inert atmosphere; | 85% |
-
-
127740-25-4
2-[(4-acetylaminophenylamino)methylene]malonic acid diethyl ester

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With ethylenediamine In ethanol at 20℃; for 1.3h; | 80% |
-
-
27514-08-5
N-(4-oxocyclohexyl)acetamide

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With ethene; 5%-palladium/activated carbon; ammonium acetate; potassium carbonate In acetonitrile at 90℃; under 760.051 Torr; for 15h; Reagent/catalyst; Schlenk technique; | 80% |
| With styrene; ammonium hydroxide In 1-methyl-pyrrolidin-2-one at 130℃; for 20h; Sealed tube; Inert atmosphere; | 65% |

| Conditions | Yield |
|---|---|
| A n/a B 76% |
-
-
16375-92-1
4-(+/-)-α-hydroxyethylphenylacetamide

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With sodium azide; trifluoroacetic acid In hexane at 40℃; for 4h; Sealed tube; | 76% |
| With sodium azide; methanesulfonic acid; trifluoroacetic acid In hexane at 40℃; for 12h; | 76% |

| Conditions | Yield |
|---|---|
| With 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate In acetonitrile at 20℃; for 5h; Irradiation; | 75% |
| With 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate In acetonitrile at 20℃; for 5h; Irradiation; | 75% |
-
-
108-24-7
acetic anhydride

-
-
106-50-3
1,4-phenylenediamine

-
A
-
140-50-1
N,N'-diacetyl-1,4-phenylenediamine

-
B
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; | A 7% B 63% |


| Conditions | Yield |
|---|---|
| With hydroxylamine triflate; iron(II) sulfate In water; acetonitrile at 20℃; for 16h; | 62% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; water; caesium carbonate; potassium hydroxide; N,N`-dimethylethylenediamine at 100℃; for 15h; Inert atmosphere; Schlenk technique; | 58% |

| Conditions | Yield |
|---|---|
| With nitromethane; trifluoromethylsulfonic anhydride; acetic acid In formic acid at 80 - 120℃; | 50% |
-
-
38063-81-9
1-(4-aminophenyl)ethanone oxime

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; tetrabutylammonium tetrafluoroborate; water In 1,2-dichloro-ethane at 20℃; for 0.733333h; Beckmann Rearrangement; Electrochemical reaction; | 31% |
-
-
101906-07-4
N-(4-Butylamino-phenyl)-acetamide

-
A
-
123-72-8
butyraldehyde

-
B
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With oxygen; salcomine In methanol for 24h; Heating; | A n/a B 24% |
-
-
104-04-1
N-(4-Nitrophenyl)acetamide

-
A
-
122-80-5
N-acetyl-p-phenylenediamine

-
B
-
97495-17-5
bis-(4-acetylamino-phenyl)-diazene-N-oxide

| Conditions | Yield |
|---|---|
| durch elektrolytische Reduktion; |
-
-
127-09-3
sodium acetate

-
-
540-24-9
p-phenylenediamine hydrochloride

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With water |
-
-
98-59-9
p-toluenesulfonyl chloride

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
27022-64-6
N-(4-(4-methylphenylsulfonamido)phenyl)acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine In N,N-dimethyl-formamide; benzene for 24h; Ambient temperature; | |
| With pyridine In dichloromethane at 20℃; |
-
-
75-15-0
carbon disulfide

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
35008-62-9
4-(N-acetylamino)phenyl isothiocyanate

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; N-acetyl-p-phenylenediamine With triethylamine In ethanol at 20℃; Stage #2: With dmap; di-tert-butyl dicarbonate In ethanol at 20℃; for 0.25h; Further stages.; | 100% |
| Stage #1: carbon disulfide; N-acetyl-p-phenylenediamine With triethylamine In ethanol at 20℃; for 1h; Stage #2: With dmap; di-tert-butyl dicarbonate at 0℃; for 2h; | |
| Stage #1: carbon disulfide; N-acetyl-p-phenylenediamine With triethylamine In tetrahydrofuran at 20℃; Stage #2: With dmap; di-tert-butyl dicarbonate In tetrahydrofuran at 0 - 20℃; | 100 %Chromat. |
| Stage #1: carbon disulfide; N-acetyl-p-phenylenediamine With triethylamine In ethanol at 20℃; for 2h; Stage #2: With dmap; di-tert-butyl dicarbonate In ethanol at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 70℃; for 5h; Microwave irradiation; Inert atmosphere; neat (no solvent); | 99% |
| With ammonium iodide; hydrazine at 50℃; for 12h; | 99% |
| With ammonium iodide; hydrazine hydrate at 50℃; for 12h; Inert atmosphere; Sealed tube; | 99% |
-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
112-82-3
hexadecanyl bromide

-
-
85074-37-9
N-(4-dihexadecylaminophenyl)acetamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In 2-methoxy-ethanol | 99% |
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 120℃; for 14h; | 82% |
-
-
83135-00-6
(hex-1-en-3-yl)methyl carbonate

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With cobalt(II) tetrafluoroborate; C36H29N2O2P; zinc In acetonitrile at 20℃; for 16h; Inert atmosphere; Sealed tube; enantioselective reaction; | 99% |
-
-
42889-86-1
5-nitro-2-furoyl isothiocyanate

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
117457-85-9
N-{4-[3-(5-Nitro-furan-2-carbonyl)-thioureido]-phenyl}-acetamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 27h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: piperonal; N-acetyl-p-phenylenediamine With Decaborane In methanol at 20℃; for 0.5h; Stage #2: formaldehyd With Decaborane In methanol; water at 20℃; for 1.5h; | 98% |


-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4-(4-(4-(((6aS)-5-((allyloxy)carbonyl)-2-methoxy-12-oxo-6-((tetrahydro-2H-pyran-2-yl)oxy)-5,6,6a,7,8,9,10,12-octahydrobenzo[e]pyrido[1,2-a][1,4] diazepin-3-yl)oxy)butanamido)-1-methyl-1H-pyrrole-2- carboxamido)phenyl)-1-methyl-1H-pyrrole-2-carboxylic acid With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 0.25h; Stage #2: N-acetyl-p-phenylenediamine In dichloromethane at 20℃; for 1.5h; | 98% |
-
-
476-60-8
leucoquinizarin

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| Stage #1: N-acetyl-p-phenylenediamine With hydrogenchloride In water at 40 - 50℃; for 0.5h; Stage #2: leucoquinizarin; 1,4-dihydroxy-9,10-anthracenedione In water at 85 - 90℃; Solvent; Temperature; | 97.13% |

-
-
770-06-9
5-nitro-3-furanoyl chloride

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
144369-59-5
5-Nitro-furan-3-carboxylic acid (4-acetylamino-phenyl)-amide

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 10 - 15℃; for 3h; | 97% |
-
-
3076-56-0
p-tolyllead triacetate

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
33089-86-0
N-{4-[(4-methylphenyl)amino]phenyl}acetamide

| Conditions | Yield |
|---|---|
| copper diacetate In dichloromethane at 25℃; for 1h; Arylation; | 97% |
-
-
118-92-3
anthranilic acid

-
-
122-51-0
orthoformic acid triethyl ester

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
24122-35-8
N-{4-[4-oxo-3(4H)-quinazolinyl]phenyl}acetamide

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate at 60℃; for 0.0333333h; | 97% |

-
-
75-05-8
acetonitrile

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
A
-
5326-57-8
N-(4-diethylamino-phenyl)-acetamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 12h; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 12h; | 97% |
-
-
67-56-1
methanol

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
7463-28-7
N'-acetyl-N,N-dimethyl-1,4-phenylenediamine

| Conditions | Yield |
|---|---|
| With C15H29IrN4(2+)*2I(1-) at 120℃; for 17h; Inert atmosphere; Schlenk technique; Sealed tube; | 97% |
-
-
42059-81-4
3-formyl-6-methylchromone

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 7h; Reflux; | 97% |
-
-
118739-52-9
5-nitro-3-furoyl isothiocyanate

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
117457-84-8
N-{4-[3-(5-Nitro-furan-3-carbonyl)-thioureido]-phenyl}-acetamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 27h; Ambient temperature; | 96% |
-
-
61941-89-7
5-(2-chlorophenyl)-2-furoyl chloride

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| With PEG-400; sodium hydroxide In dichloromethane at 20℃; for 1h; Acylation; | 96% |
-
-
13675-94-0
4-chloro-6-nitroquinoline

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
1020150-27-9
4-[4-(acetylamino)anilino]-6-nitroquinolinium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; ethanol for 0.5h; Heating / reflux; | 96% |
-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
127740-25-4
2-[(4-acetylaminophenylamino)methylene]malonic acid diethyl ester

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 95% |
-
-
30186-41-5
1-(4-nitrophenyl)pyrrole-2-carbaldehyde

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
122275-92-7
N-(4-{[1-[1-(4-Nitro-phenyl)-1H-pyrrol-2-yl]-meth-(E)-ylidene]-amino}-phenyl)-acetamide

| Conditions | Yield |
|---|---|
| With sodium sulfate In benzene for 20h; Heating; | 95% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
141214-23-5
1-(4-Acetylamino-phenyl)-4-(4-acetylamino-phenylamino)-2-methyl-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 25℃; for 1h; Irradiation; | 95% |
-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
339274-37-2
tert-butylcarbamoyl-thioacetic acid S-tert-butyl ester

| Conditions | Yield |
|---|---|
| With silver trifluoroacetate In 1,2-dimethoxyethane at 20℃; | 95% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
769121-30-4
(4-acetylaminophenyl)carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With zinc(II) perchlorate In tert-butyl alcohol at 20℃; for 72h; | 95% |
| With PEG-400 at 20℃; for 2h; Neat (no solvent); chemoselective reaction; | 86% |
| With 1-butyl-3-methylimidazolium trifluoromethanesulfonimide at 30 - 35℃; for 0.75h; neat (no solvent); chemoselective reaction; | 80% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
579516-14-6
N-(4-(2-chloropyrimidin-4-ylamino)phenyl)acetamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; ethanol at 75℃; | 95% |
| With sodium hydrogencarbonate In tetrahydrofuran; ethanol at 0 - 20℃; for 16h; |
-
-
15009-92-4
2,5-dinitropyridine

-
-
122-80-5
N-acetyl-p-phenylenediamine

-
-
801243-60-7
N-[4-(5-nitropyridin-2-ylamino)phenyl]acetamide

| Conditions | Yield |
|---|---|
| In ethanol; acetic acid at 20℃; for 2h; | 95% |
4'-Aminoacetanilide Specification
The p-Amino acetanilide with CAS registry number of 122-80-5 is also called Acetamide,N-(4-aminophenyl)-. The IUPAC name is N-(4-aminophenyl)acetamide. Its EINECS registry number is 204-576-6. In addition, the molecular formula is C8H10N2O and the molecular weight is 150.1778. It belongs to the classes of Amineprimary; Intermediates of Dyes and Pigments; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts. It is a kind of white or slightly reddish solid. And it is slightly soluble in water. What's more, it should be stored in a airtight, cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 0.08; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0; (4)ACD/LogD (pH 7.4): 0; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 22; (8)ACD/KOC (pH 7.4): 26; (9)#H bond acceptors: 3; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 55.12 Å2; (13)Index of Refraction: 1.636; (14)Molar Refractivity: 44.761 cm3; (15)Molar Volume: 124.796 cm3; (16)Polarizability: 17.745 ×10-24cm3; (17)Surface Tension: 53.079 dyne/cm; (18)Density: 1.203 g/cm3; (19)Flash Point: 115.717 °C; (20)Enthalpy of Vaporization: 50.577 kJ/mol; (21)Boiling Point: 267.726 °C at 760 mmHg; (22)Vapour Pressure: 0.008 mmHg at 25°C.
Preparation of p-Amino acetanilide: it can be prepared by p-nitroacetylaniline. p-Nitroacetylaniline can be prepared by acetylaniline. At first, you can put the sulfuric acid into the kettle, then add the acetylaniline in 2-2.5 hours with stirring at the temperature of 20-25 °C and make the whole to dissolve. Then cool the mixture to 4-7 ℃, add mix acid in 20 hours by dropping. After the separation process, you can get p-nitroacetylaniline. At last, you can use iron powder to resolve p-nitroacetylaniline to get p-Amino acetylaniline.
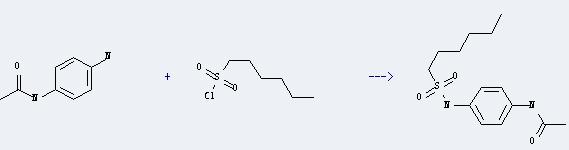
Uses of p-Amino acetanilide: it can be used in organic synthesis and pharmaceutical industry. it can be used as Azo Dye Intermediates. It can react with hexane-1-sulfonyl chloride to get N-(4-acetamidophenyl)sulfamoylhexane. This reaction will need reagent pyridine. The yield is about 83%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes and harmful by inhalation, in contact with skin and if swallowed. And it may cause sensitization by inhalation and skin contact. During using it, you should not breathe dust and should wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Nc1ccc(NC(C)=O)cc1
(2)InChI: InChI=1/C8H10N2O/c1-6(11)10-8-4-2-7(9)3-5-8/h2-5H,9H2,1H3,(H,10,11)
(3)InChIKey: CHMBIJAOCISYEW-UHFFFAOYAK
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 633mg/kg (633mg/kg) | Meditsina Truda i Promyshlennaya Ekologiya. Industrial Medicine and Ecology. Vol. (10), Pg. 36, 1996. | |
| rat | LD50 | oral | 2500mg/kg (2500mg/kg) | Meditsina Truda i Promyshlennaya Ekologiya. Industrial Medicine and Ecology. Vol. (10), Pg. 36, 1996. |
Related Products
- 4-Aminoacetanilide-3-sulfonic acid
- 122813-72-3
- 1228175-65-2
- 1228182-56-6
- 122818-32-0
- 1228183-22-9
- 1228244-79-8
- 122-82-7
- 122836-35-5
- 122839-48-9
- 122839-50-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View