-
Name
4-Aminopyridine
- EINECS 207-987-9
- CAS No. 504-24-5
- Article Data144
- CAS DataBase
- Density 1.107 g/cm3
- Solubility Soluble in water, ethanol, slightly soluble in ethyl ether and benzene, insoluble in light oil and petroleum
- Melting Point 157 °C
- Formula C5H6N2
- Boiling Point 255.2 °C at 760 mmHg
- Molecular Weight 94.116
- Flash Point 131.8 °C
- Transport Information UN 2671 6.1/PG 2
- Appearance white to slightly yellow fine crystalline powder
- Safety 26-36/37/39-45-60-61
- Risk Codes 36/37/38-51/53-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, N,
N, Xi
Xi
- Synonyms Pyridin-4-amine;1H-Pyridin-4-amine;Amino-4 pyridine;gamma-Aminopyridine;p-Aminopyridine;Fampridine;Pyridine, 4-amino-;p-Aminopyridine;Pimadin (free base);
- PSA 38.91000
- LogP 1.24500
Synthetic route
-
-
98400-69-2
N-(4-pyridyl) t-butyl carbamate

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With nitric acid In dichloromethane at 0℃; for 2h; | 97% |

| Conditions | Yield |
|---|---|
| With C36H56Cl3CrN2O; magnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; chemoselective reaction; | 97% |
| With hydrogen In water at 25℃; for 15h; Green chemistry; chemoselective reaction; | 94% |
| With triethylamine In water at 80℃; for 6h; Reagent/catalyst; Solvent; Inert atmosphere; Green chemistry; chemoselective reaction; | 92% |
-
-
15854-87-2
4-iodopyridine

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With copper(l) iodide; ascorbic acid In ammonia at 25℃; for 18h; liquid NH3; | 97% |

| Conditions | Yield |
|---|---|
| With ammonia; copper dichloride at 65℃; for 8h; Temperature; Autoclave; Inert atmosphere; | 96.7% |
| With ammonia; zinc(II) chloride at 220 - 230℃; im Rohr; | |
| Multi-step reaction with 2 steps 1: 2 h / Heating 2: CH3SO2OH / 10percent Pd-C / ethanol / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; N-ethyl-N,N-diisopropylamine In water; acetonitrile at 30℃; for 4h; Solvent; | 96% |
| With sodium hypochlorite; sodium hydroxide at 0 - 10℃; | 91.8% |
| With potassium hydroxide; bromine at 70℃; | |
| With chlorine; sodium hydroxide In water at -5 - 5℃; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; tin(ll) chloride In tetrahydrofuran for 0.5h; Ambient temperature; | 95% |
| With palladium 10% on activated carbon; ammonium formate In ethanol at 20℃; for 16h; | 94% |
| With sodium hypophosphite; palladium on activated charcoal In acetic acid at 60℃; for 2.5h; | 92% |
-
-
13556-71-3
N-benzyl-4-aminopyridine

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With ammonium formate; zinc In ethylene glycol for 0.0416667h; microwave irradiation; | 95% |
| With ammonium formate; magnesium In ethylene glycol for 0.025h; microwave irradiation; | 95% |
| With sulfuric acid for 24h; Ambient temperature; | 78% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; sodium hydroxide In water at 40 - 70℃; for 1h; Product distribution / selectivity; | 90.8% |
| With sodium hypochlorite; sodium hydroxide In water at 40 - 70℃; for 1h; Product distribution / selectivity; | 90.8% |
| With sodium hypochlorite; sodium pertungstate In water at 0 - 95℃; for 12h; Temperature; |

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In dichloromethane at 40 - 45℃; for 4h; | 90.6% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; zirconium(IV) chloride In tetrahydrofuran at 0 - 35℃; for 0.25h; Reduction; | 90% |
| With sodium tetrahydroborate; lithium chloride In tetrahydrofuran at 35℃; for 0.5h; Reduction; | 90% |
| With palladium on activated charcoal; ethanol Hydrogenation; | |
| With methanol; nickel Hydrogenation; |
-
-
1124-33-0
4-nitraminopyridine N-oxide

-
A
-
504-24-5
4-aminopyridine

-
B
-
3535-75-9
4-aminopyridine-1-oxide

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene; 5%-palladium/activated carbon In methanol at 120℃; for 0.0833333h; Microwave irradiation; | A 90% B 10% |
-
-
39910-67-3
4-azidopyridine

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With iron In water at 20℃; Inert atmosphere; | 88% |
| With C36H44CuN8(1+)*BF4(1-) In water; toluene at 100℃; for 20h; Sealed tube; chemoselective reaction; | 45% |
| With water for 5h; Inert atmosphere; UV-irradiation; Sealed tube; chemoselective reaction; | 44% |
| With 2,6-di-tert-butyl-4-methyl-phenol In decalin at 153.8℃; Kinetics; Rate constant; Thermodynamic data; Eact, ΔSact; var. temper.; var. conc. of inhibitor; |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; CYANAMID; bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; for 1h; chemoselective reaction; | 85% |
| With N-Bromosuccinimide; N-methoxylamine hydrochloride; bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; | 78% |

| Conditions | Yield |
|---|---|
| In formamide at 130 - 150℃; for 20h; | 79% |
| Stage #1: 4-pyridinecarbohydroxamic acid With potassium carbonate In dimethyl sulfoxide at 90℃; for 3h; Lossen Rearrangement; Stage #2: With hydrogenchloride In water; dimethyl sulfoxide at 20℃; for 1h; |
-
-
19524-06-2
4-bromopyridine hydrochloride

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; lithium hexamethyldisilazane In toluene at 100℃; for 4h; Glovebox; Sealed tube; | 78% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; L-2-O-methyl-chiro-inositol; copper(II) acetate monohydrate In 1-methyl-pyrrolidin-2-one at 110℃; for 30h; | 75% |
| With copper(I) oxide; ammonium hydroxide In 1-methyl-pyrrolidin-2-one at 80℃; for 24h; | 74% |
| With ammonia; water at 200℃; |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; 5%-palladium/activated carbon; hydrazine hydrate; lithium hydroxide In 1,4-dioxane at 170℃; for 16h; Molecular sieve; Inert atmosphere; | 52% |

| Conditions | Yield |
|---|---|
| for 6h; Reflux; neat (no solvent); | 51% |

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-(1,3-dithian-2-yl)propan-2-yl (pyridin-4-yl)carbamate With sodium periodate In tetrahydrofuran; water at 20℃; for 12h; Inert atmosphere; Schlenk technique; Stage #2: With potassium carbonate In methanol at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 48% |
-
-
1135-35-9
cyclohexanone-[4]pyridylhydrazone

-
-
141-52-6
sodium ethanolate

-
A
-
504-24-5
4-aminopyridine

-
B
-
4329-69-5
6,7,8,9-tetrahydro-5H-pyrido[4,3-b]indole

-
C
-
35036-85-2
N-ethylpyridine-4-amine

| Conditions | Yield |
|---|---|
| In diethylene glycol at 235 - 240℃; for 0.25h; | A 15% B 39% C 37% |

-
-
1135-35-9
cyclohexanone-[4]pyridylhydrazone

-
A
-
504-24-5
4-aminopyridine

-
B
-
4329-69-5
6,7,8,9-tetrahydro-5H-pyrido[4,3-b]indole

-
C
-
35036-85-2
N-ethylpyridine-4-amine

| Conditions | Yield |
|---|---|
| With sodium ethanolate In diethylene glycol at 235 - 240℃; for 0.25h; | A 15% B 39% C 37% |
| With sodium ethanolate In diethylene glycol at 235 - 240℃; for 0.25h; Rate constant; Product distribution; other solvent, various concentrations of sodium ethoxide; | A 15% B 39% C 37% |

-
-
2569-58-6, 54772-94-0, 54773-16-9
(E)-4-phenylazopyridine

-
A
-
504-24-5
4-aminopyridine

-
B
-
123-30-8
4-amino-phenol

-
C
-
20815-66-1
4-(2-(pyridin-4-yl)diazenyl)phenol

| Conditions | Yield |
|---|---|
| With sulfuric acid Product distribution; Rate constant; reaction of phenylazopyridines with aq. H2SO4; reaction intermediates, kinetics and mechanism; effect of H2SO4 concentration of product ratio and reaction rate; UV study; | A 19% B 20% C 22% D 31% |
| With sulfuric acid | A 19% B 20% C 22% D 31% |


| Conditions | Yield |
|---|---|
| With 4 A molecular sieve at 500℃; under 0.1 Torr; Product distribution; flash vaccum pyrolysis; | A 26.1% B n/a C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| With 4 A molecular sieve at 500℃; under 0.1 Torr; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; | A 26.1% B n/a C n/a D n/a |

-
-
51790-32-0
4-(N'-Pyridin-4-yl-hydrazino)-phenol

-
A
-
504-24-5
4-aminopyridine

-
B
-
123-30-8
4-amino-phenol

-
C
-
20815-66-1
4-(2-(pyridin-4-yl)diazenyl)phenol

| Conditions | Yield |
|---|---|
| With sulfuric acid | A 22% B 24% C 26% |
| With sulfuric acid Product distribution; Rate constant; reaction of phenylazopyridines with aq. H2SO4; reaction intermediates, kinetics and mechanism; effect of H2SO4 concentration of product ratio and reaction rate; UV study; | A 22% B 24% C 26% |
| With sulfuric acid Product distribution; Rate constant; acid-catalysed disproportionation and benzidine rearrangement of phenylhydrazinopyridines; reaction pathways; kinetics and mechanism; | A 22% B 24% C 26% |

-
-
20815-53-6
4-(4-chlorophenylazo)pyridine

-
A
-
504-24-5
4-aminopyridine

-
D
-
17609-80-2
4-amino-3-chlorophenol

-
E
-
554-00-7
2,4-Dichloroaniline

-
F
-
253333-13-0
(E)-4-(pyridin-4-yldiazenyl)phenol

| Conditions | Yield |
|---|---|
| With sulfuric acid Rate constant; Product distribution; var. conc. of acid; | A 26% B 13% C 15% D 14% E 10% F 2% |


-
A
-
504-24-5
4-aminopyridine

-
C
-
554-00-7
2,4-Dichloroaniline

-
D
-
106-47-8
4-chloro-aniline

-
F
-
20815-53-6
4-(4-chlorophenylazo)pyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid for 24h; Rate constant; Mechanism; Product distribution; var. conc. of acid; var. time; other (arylazo)pyridine; | A 25% B 21% C 20% D 3% E n/a F 5% |

-
-
72109-70-7
4-(2-phenylhydrazin)pyridine

-
A
-
504-24-5
4-aminopyridine

-
B
-
123-30-8
4-amino-phenol

-
C
-
60172-08-9
N1-(4-pyridinyl)-1,4-benzenediamine

-
D
-
20815-66-1
4-(2-(pyridin-4-yl)diazenyl)phenol

-
E
-
2569-58-6, 54772-94-0, 54773-16-9
(E)-4-phenylazopyridine

-
F
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With sulfuric acid Product distribution; Rate constant; acid-catalysed disproportionation and benzidine rearrangement of phenylhydrazinopyridines; reaction pathways; kinetics and mechanism; | A 15% B 7% C 6% D 5% E 9% F 8% |

-
-
72109-70-7
4-(2-phenylhydrazin)pyridine

-
A
-
504-24-5
4-aminopyridine

-
B
-
60172-08-9
N1-(4-pyridinyl)-1,4-benzenediamine

-
C
-
2569-58-6, 54772-94-0, 54773-16-9
(E)-4-phenylazopyridine

-
D
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With sulfuric acid Further byproducts given; | A 15% B 6% C 9% D 8% |

-
-
504-24-5
4-aminopyridine

-
-
1943-83-5
2-chloroethyl isothiocyanate

-
-
62491-96-7
N-(2-chloroacetyl)-N'-(4-pyridyl)urea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; | 100% |
| In toluene at 0 - 12℃; for 12h; | 94% |
| In toluene at 20℃; for 6h; | 87% |
-
-
504-24-5
4-aminopyridine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
98400-69-2
N-(4-pyridyl) t-butyl carbamate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) at 30 - 35℃; for 0.5h; | 100% |
| With perchloric acid at 30 - 35℃; for 0.25h; | 100% |
| In PEG-400 at 20℃; for 0.25h; | 100% |
-
-
864950-69-6
methanesulfonic acid 3-(2-phenylethynyl-phenyl)-prop-2-ynyl ester

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In water; dimethyl sulfoxide at 25℃; Kinetics; | 100% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 120℃; for 1h; | 100% |
| Heating; |

| Conditions | Yield |
|---|---|
| In various solvent(s) for 24h; Heating; | 100% |
-
-
504-24-5
4-aminopyridine

-
-
627906-79-0
4-[2-(11-ethyl-6,11-dihydro-5-methyl-6-oxo-5H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-8-yl)ethoxy]-3-methylbenzoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25℃; for 5h; | 100% |
-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With fluorosilicic acid In methanol | 100% |
-
-
504-24-5
4-aminopyridine

-
-
13160-58-2
4-nitro-N-(pyridin-4-yl)benzamide

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 0.0333333h; Flow reactor; | 100% |
-
-
504-24-5
4-aminopyridine


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 100% |
| With N-[(dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 0.0833333h; Menshutkin Reaction; | 100% |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; 1,1'-bis-(diphenylphosphino)ferrocene; tris(dibenzylideneacetone)dipalladium (0) In toluene at 115℃; for 18h; | 99.9% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate In toluene at 120℃; for 18h; Sealed tube; | 99% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; sodium t-butanolate; tris(dibenzylideneacetone)dipalladium (0) In toluene at 100℃; for 14h; Cross-coupling; | 96% |

| Conditions | Yield |
|---|---|
| for 8h; | 99% |
| In ethyl acetate | |
| In diethyl ether at 20℃; | |
| In acetonitrile at 80℃; |
-
-
504-24-5
4-aminopyridine

-
-
181823-46-1
2-(2-Dimethylamino-benzylsulfanyl)-nicotinic acid

-
-
181822-33-3
2-(2-Dimethylamino-benzylsulfanyl)-N-pyridin-4-yl-nicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 3h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; | 99% |
| In tetrahydrofuran at 25℃; for 3h; | 78.6% |
-
-
504-24-5
4-aminopyridine

-
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); trifuran-2-yl-phosphane; caesium carbonate In toluene at 90℃; under 760 Torr; for 18h; Michael addition; | 99% |

-
-
865094-47-9
isopropenyl (5-tert-butyl-2-(p-tolyl)-2H-pyrazol-3-yl)carbamate

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| With 1-Methylpyrrolidine In tetrahydrofuran at 55℃; for 0.5h; | 99% |

-
-
504-24-5
4-aminopyridine

-
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0) In toluene at 90℃; for 18h; | 99% |

-
-
504-24-5
4-aminopyridine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
75-65-0
tert-butyl alcohol

-
-
98400-69-2
N-(4-pyridyl) t-butyl carbamate

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water | 99% |

-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| In methanol N2; Co comp. dissolved under reflux, to a soln. added an excess of ligand, refluxed for 25 min, a soln. of NH4PF6 added; ppt. filtered, washed copiously (diethyl ether), dried (vac.); elem. anal.; | 99% |

-
-
504-24-5
4-aminopyridine

-
-
736141-91-6, 195193-91-0
trans-bis[O-methyl-(4-methoxyphenyl)phosphonodithioato]nickel

-
-
377737-43-4
[Ni(O-methyl-(4-methoxyphenyl)phosphonodithioato)2(p-aminopyridine)2]

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane excess of pyridine deriv. in MeOH was added dropwise to soln. of Ni complex in CH2Cl2, slow evapn. of mixt. at room temp.; elem. anal.; | 99% |
-
-
504-24-5
4-aminopyridine

| Conditions | Yield |
|---|---|
| In methanol excess of N-compd. was added to hot MeOH soln. of Cu-complex, reflux for25 min, concd. MeOH soln. of NH4PF6 was added; ppt. was collected, washed with copious amt. of Et2O, dried in vac., elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With iron(II,III) oxide; potassium tert-butylate In 1,4-dioxane at 90℃; for 168h; Inert atmosphere; | 99% |
| With cesium hydroxide; (sat-NHC-Bn)Ir(CO)(PPh3)Cl at 100℃; for 24h; Inert atmosphere; | 99% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; | 99% |
-
-
504-24-5
4-aminopyridine

-
-
779355-57-6
(pyridine)2Ni{CH2CH2C(=O)O}

-
-
1232169-66-2
[Ni(4-aminopyridine)2(C2H4COO)]

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide byproducts: py; Ar; DMF soln. of Ni compd. (2.04 mmol) treated with ligand (4.14 mmol), mixt. stirred for 30 min; evapd., elem. anal.; | 99% |
-
-
504-24-5
4-aminopyridine

-
-
495-76-1
piperonol

-
-
1301628-37-4
N-(benzo[d][1,3]dioxol-5-ylmethyl)pyridin-4-amine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; Inert atmosphere; | 99% |
| With cesium hydroxide; palladium diacetate In toluene at 150℃; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| In acetone for 16h; Inert atmosphere; Schlenk technique; | 99% |
| In acetone at 20℃; for 12h; | 95% |

| Conditions | Yield |
|---|---|
| at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 48h; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 48h; chemoselective reaction; | 99% |
4-Aminopyridine Consensus Reports
4-Aminopyridine Standards and Recommendations
4-Aminopyridine Specification
The 4-Aminopyridine, with the CAS registry number 504-24-5, is also known as Dalfampridine. 4-Aminopyridine is an organic compound with the chemical formula C5H4N–NH2. It belongs to the product categories of Pyridine; Organics; Amines; Pyridines; Pyridines derivates; Heterocyclic Compounds; Alphabetical Listings; Stable Isotopes; Potassium channel; Ion Channels. Its EINECS number is 207-987-9 and molecular weight is 94.11. What's more, its systematic name is pyridin-4-amine. It should be sealed and stored in a dry place. Moreover, it should be protected from light. It is one of the potassium channel blockers, with secondary effect on calcium currents, which is used mainly as a research tool and to characterize channel subtypes. It is used in organic synthesis.
Physical properties of 4-Aminopyridine are: (1)ACD/LogP: 0.26; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.22; (4)ACD/LogD (pH 7.4): -1.51; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 16.13 Å2; (13)Index of Refraction: 1.587; (14)Molar Refractivity: 28.58 cm3; (15)Molar Volume: 84.9 cm3; (16)Polarizability: 11.33×10-24cm3; (17)Surface Tension: 51.2 dyne/cm; (18)Density: 1.107 g/cm3; (19)Flash Point: 131.8 °C; (20)Enthalpy of Vaporization: 49.27 kJ/mol; (21)Boiling Point: 255.2 °C at 760 mmHg; (22)Vapour Pressure: 0.0165 mmHg at 25°C.
Preparation of 4-Aminopyridine: this chemical can be prepared by 4-nitro-pyridine-1-oxide at the ambient temperature. This reaction will need reagents TiCl4, SnCl2 and solvent tetrahydrofuran with the reaction time of 30 min. The yield is about 95%.

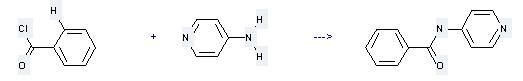
Uses of 4-Aminopyridine: it can be used to produce N-pyridin-4-yl-benzamide at the temperature of 60 °C. It will need reagent triethylamine and solvent 1,2-dichloro-ethane with the reaction time of 3 hours. The yield is about 73%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic by inhalation, in contact with skin and if swallowed. It is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). This material and its container must be disposed of as hazardous waste. You should avoid releasing it to the environment just refering to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: n1ccc(N)cc1
(2)Std. InChI: InChI=1S/C5H6N2/c6-5-1-3-7-4-2-5/h1-4H,(H2,6,7)
(3)Std. InChIKey: NUKYPUAOHBNCPY-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 2370ug/kg (2.37mg/kg) | ASTM Special Technical Publication. Vol. (680), Pg. 157, 1979. | |
| dog | LD50 | oral | 3700mg/kg (3700mg/kg) | Toxicology and Applied Pharmacology. Vol. 26, Pg. 532, 1973. | |
| duck | LD50 | oral | 4200mg/kg (4200mg/kg) | Toxicology and Applied Pharmacology. Vol. 21, Pg. 315, 1972. | |
| man | LDLo | oral | 590ug/kg (.59mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Clinical Toxicology. Vol. 16, Pg. 487, 1980. |
| mouse | LD50 | intracrebral | 4mg/kg (4mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Australian Journal of Experimental Biology and Medical Science. Vol. 36, Pg. 365, 1958. |
| mouse | LD50 | intraperitoneal | 10mg/kg (10mg/kg) | Journal of Medicinal Chemistry. Vol. 8, Pg. 296, 1965. | |
| mouse | LD50 | intravenous | 7mg/kg (7mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Annales Pharmaceutiques Francaises. Vol. 26, Pg. 345, 1968. |
| mouse | LD50 | oral | 19mg/kg (19mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 57(9-10), Pg. 64, 1992. | |
| mouse | LDLo | subcutaneous | 5mg/kg (5mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 226, Pg. 163, 1955. | |
| pigeon | LD50 | oral | 6mg/kg (6mg/kg) | Journal of Wildlife Management. Vol. 29, Pg. 830, 1965. | |
| quail | LD50 | oral | 7650ug/kg (7.65mg/kg) | ASTM Special Technical Publication. Vol. (680), Pg. 157, 1979. | |
| rabbit | LD50 | intravenous | 5500ug/kg (5.5mg/kg) | Eksperimentalna Meditsina i Morfologiya. Vol. 11, Pg. 162, 1972. | |
| rat | LD50 | intraperitoneal | 6500ug/kg (6.5mg/kg) | Toxicology and Applied Pharmacology. Vol. 26, Pg. 532, 1973. | |
| rat | LD50 | oral | 21mg/kg (21mg/kg) | Journal de Toxicologie Clinique et Experimentale. Vol. 6(3), Pg. 175, 1986. | |
| rat | LD50 | subcutaneous | 19mg/kg (19mg/kg) | Eksperimentalna Meditsina i Morfologiya. Vol. 11, Pg. 162, 1972. | |
| women | TDLo | oral | 120ug/kg (.12mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Toxicology, Clinical Toxicology. Vol. 32, Pg. 583, 1994. |
Related Products
- 4-Aminopyridine
- 4-Aminopyridine 1-oxide
- 4-Aminopyridine hydrochloride
- 4-Aminopyridine-2-carbonitrile
- 4-Aminopyridine-2-carboxylic acid
- 4-Aminopyridine-3-carboxylic acid ethyl ester
- 50424-81-2
- 5042-53-5
- 50427-77-5
- 50427-78-6
- 504-29-0
- 504-30-3
- 50-43-1
- 50432-68-3
- 50432-79-6
- 50433-06-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View