-
Name
Calcium sulfide
- EINECS
- CAS No. 20548-54-3
- Density g/cm3
- Solubility
- Melting Point 2000 °C
- Formula CaS
- Boiling Point °Cat760mmHg
- Molecular Weight 72.14
- Flash Point °C
- Transport Information
- Appearance OFF-WHITE POWDER
- Safety A poison via inhalation. Reacts violently with chromyl chloride, lead dioxide, potassium chlorate (mild explosion), potassium nitrate (violent explosion). Incompatible with oxidants. When heated to decomposition it emits toxic fumes of SOx. See also CALCIUM COMPOUNDS and SULFIDES.
- Risk Codes R31; R36/37/38; R50
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, N
N
- Synonyms Calciumsulfide (8CI); Baumol; C.I. 77245; C.I. Pigment White 9; Calcium monosulfide;Pigment White 9
- PSA 25.30000
- LogP -0.53540
CALCIUM SULFIDE Chemical Properties
The Molecular formula of Calcium sulfide(20548-54-3): CaS
The Molar mass of Calcium sulfide(20548-54-3): 72.143 g/mol
Structure: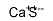
Appearance: white crystals hygroscopic
Density: 2.59 g/cm3
Melting point: 2525 °C
Solubility in water: slightly soluble
Solubility: insoluble in alcohol;reacts with acid
Refractive index (nD): 2.137
Other names: Calcium monosulfide,Hepar calcies,Sulfurated lime,Oldhamite.
Calcium sulfide (20548-54-3) is the chemical compound with the formula CaS. Calcium sulfide (20548-54-3) is a white material that crystallizes in cubes like rock salt. Like many salts containing sulfide ions, Calcium sulfide (20548-54-3) typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.
The Molar mass of Calcium sulfide(20548-54-3): 72.143 g/mol
Structure:
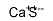
Appearance: white crystals hygroscopic
Density: 2.59 g/cm3
Melting point: 2525 °C
Solubility in water: slightly soluble
Solubility: insoluble in alcohol;reacts with acid
Refractive index (nD): 2.137
Other names: Calcium monosulfide,Hepar calcies,Sulfurated lime,Oldhamite.
Calcium sulfide (20548-54-3) is the chemical compound with the formula CaS. Calcium sulfide (20548-54-3) is a white material that crystallizes in cubes like rock salt. Like many salts containing sulfide ions, Calcium sulfide (20548-54-3) typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.
CALCIUM SULFIDE Uses
Calcium sulfide(20548-54-3) is used as a depilatory in the tanning industry and in cosmetics and in a finely divided form, it is employed in luminous paints.
Calcium sulfide(20548-54-3) is used in homeopathy.
CALCIUM SULFIDE Production
Calcium sulfide(20548-54-3) is made by reducing calcium sulfate with coke.
CaSO4 + 2C → CaS + 2CO2
CaSO4 + 2C → CaS + 2CO2
CALCIUM SULFIDE Safety Profile
A poison via inhalation. Reacts violently with chromyl chloride, lead dioxide, potassium chlorate (mild explosion), potassium nitrate (violent explosion). Incompatible with oxidants. When heated to decomposition it emits toxic fumes of SOx. See also CALCIUM COMPOUNDS and SULFIDES.
Hazard Codes: Xi,
Xi, N
N
Risk Statements: 31-36/37/38-50
31: Contact with acids liberates toxic gas
36/37/38: Irritating to eyes, respiratory system and skin
50: Very Toxic to aquatic organisms
Safety Statements: 28-61
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
61: Avoid release to the environment. Refer to special instructions safety data sheet
RIDADR: UN 3179 4.1/PG 2
WGK Germany: 2
HazardClass: 4.3
PackingGroup: III
Hazard Codes:
 Xi,
Xi, N
N Risk Statements: 31-36/37/38-50
31: Contact with acids liberates toxic gas
36/37/38: Irritating to eyes, respiratory system and skin
50: Very Toxic to aquatic organisms
Safety Statements: 28-61
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
61: Avoid release to the environment. Refer to special instructions safety data sheet
RIDADR: UN 3179 4.1/PG 2
WGK Germany: 2
HazardClass: 4.3
PackingGroup: III
CALCIUM SULFIDE Specification
Calcium polysulfides(CaSn), such as calcium disulfide(CaS2) and calcium pentasulfide (CaS5), are made by heating sulfur and calcium hydroxide.
Ca(OH)2 + S → CaSn + H2O + SOx
The calcium polysulfides are used as fungicides.
Ca(OH)2 + S → CaSn + H2O + SOx
The calcium polysulfides are used as fungicides.
Related Products
- Calcium
- Calcium (1)-bis(2-hydroxy-4-methylvalerate)
- Calcium (2S)-2-[(4-chlorobenzoyl)amino]-3-(1H-indol-3-yl)propanoate
- Calcium 2,4-dichlorophenolate
- Calcium 2-ethylhexanoate
- Calcium 2-hydroxypropanoate
- Calcium 2-methoxyethoxide
- Calcium 2-oxoglutarate
- Calcium 3-methyl-2-oxovalerate
- Calcium 4-aminosalicylate
- 20548-62-3
- 20549-47-7
- 205495-66-5
- 20551-25-1
- 205517-34-6
- 205518-89-4
- 205524-46-5
- 205526-22-3
- 205526-23-4
- 205526-24-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View