-
Name
Cupric oxalate
- EINECS 212-411-4
- CAS No. 5893-66-3
- Density 1.28g/cm3
- Solubility
- Melting Point 310°C
- Formula CuC2O4
- Boiling Point 365.1 °C at 760 mmHg
- Molecular Weight 151.56
- Flash Point 188.8 °C
- Transport Information 3288
- Appearance Blue fine powder
- Safety 36
- Risk Codes 20/21/22
-
Molecular Structure
- Hazard Symbols
- Synonyms Cupric oxalate
- PSA 52.60000
- LogP -1.00110
Cupric oxalate Chemical Properties
IUPAC Name: Copper oxalate
Molecular Formula: C2CuO4
Molecular Weight: 151.57 g/mol
Molecular Structure of Copper oxalate (CAS NO.814-91-5):
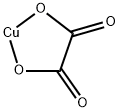
Flash Point: 188.8 °C
Boiling Point: 365.1 °C at 760 mmHg
Enthalpy of Vaporization: 67.15 kJ/mol
Appearance: Odorless bluish-white solid.
Vapour Pressure: 2.51E-06 mmHg at 25 °C
Water Solubility: insoluble
H-Bond Acceptor: 4
Exact Mass: 150.90926
MonoIsotopic Mass: 150.90926
Topological Polar Surface Area: 80.3
Heavy Atom Count: 7
Canonical SMILES: C(=O)(C(=O)[O-])[O-].[Cu+2]
InChI: InChI=1S/C2H2O4.Cu/c3-1(4)2(5)6;/h(H,3,4)(H,5,6);/q;+2/p-2
InChIKey: QYCVHILLJSYYBD-UHFFFAOYSA-L
EINECS: 212-411-4
Product Categories: organic-metal salt.
Cupric oxalate Uses
Copper oxalate (CAS NO.814-91-5) is used as a catalysts for organic reactions.
Cupric oxalate Safety Profile
Risk Statements: 20/21/22
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 36
S36:Wear suitable protective clothing.
RIDADR: UN 2449
HazardClass: 6.1(b)
PackingGroup: III
Explodes weakly when heated slightly. See also OXALATES and COPPER COMPOUNDS.
Cupric oxalate Standards and Recommendations
DOT Classification: 6.1; Label: KEEP AWAY FROM FOOD
Cupric oxalate Specification
Copper oxalate (CAS NO.814-91-5) is also named as Caswell No. 248A ; Copper oxalate (CuC2O4) ; Copper(II) oxalate ; Cupric oxalate ; Cupric oxalate (1:1) ; EPA Pesticide Chemical Code 023305 ; Ethanedioic acid copper salt ; Ethanedioic acid, copper(2+) salt (1:1) ; HSDB 265 ; NSC 112246 ; NSC 86015 ; Oxalic acid copper(2+) salt (1:1) ; Copper, (ethanedioato(2-)-kappaO1,kappaO2)- . Copper oxalate (CAS NO.814-91-5) is odorless bluish-white solid. Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. It can serve as a reducing agent in reactions that generate carbon dioxide. Inhalation causes irritation of nose and throat. Ingestion of very large amounts may produce symptoms of oxalate poisoning; watch for edema of the glottis and delayed constriction of esophagus. Contact with eyes causes irritation. It may form toxic carbon monoxide gas in fire.
Related Products
- Cupric acetate
- Cupric acetate monohydrate
- Cupric acetylacetonate
- Cupric bromide
- Cupric carbonate
- Cupric carbonate basic
- Cupric chloride
- Cupric chloride dihydrate
- Cupric citrate
- Cupric fluoride
- 589-38-8
- 589-41-3
- 589-43-5
- 58944-75-5
- 5894-59-7
- 58-94-6
- 5894-60-0
- 5894-65-5
- 5894-79-1
- 5895-35-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View