-
Name
Cyclopentadecanolide
- EINECS 203-354-6
- CAS No. 106-02-5
- Article Data122
- CAS DataBase
- Density 0.885 g/cm3
- Solubility Soluble in six volume 80% or 90% ethanol
- Melting Point 34-38 °C(lit.)
- Formula C15H28O2
- Boiling Point 344.797 °C at 760 mmHg
- Molecular Weight 240.386
- Flash Point 143.392 °C
- Transport Information
- Appearance white crystalline low melting solid
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pentadecanoicacid, 15-hydroxy-, x-lactone (6CI,7CI);1,15-Pentadecanolide;1-Oxacyclohexadecan-2-one;15-Hydroxypentadecanoic acid lactone;15-Pentadecanolide;15-Pentadodecanolactone;2-Pentadecalone;CPE 215;Oxacyclohexadecan-2-one;Exaltolide;Muskalactone;NSC 36763;Pentadecalactone;Pentadecanolactone;Pentadecanolide;Pentalide;Thibetolide;cpd Supra;w-Pentadecalactone;
- PSA 26.30000
- LogP 4.61450
Synthetic route

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide | 100% |
-
-
4617-33-8
15-hydroxylpentadecanoic acid

-
A
-
106-02-5
pentadecanolide

-
B
-
659-76-7
1,17-dioxa-cyclodotriacontane-2,18-dione

| Conditions | Yield |
|---|---|
| With p-nitrobenzoic anhydride; scandium tris(trifluoromethanesulfonate) In tetrahydrofuran; acetonitrile Heating; Yields of byproduct given; | A 99% B n/a |
| With dmap; 2-methyl-6-nitrobenzoic anhydride In dichloromethane at 20℃; for 15h; | A 92% B 1% |
| With dmap; di-2-thienyl carbonate; hafnium(IV) trifluoromethanesulfonate In toluene; acetonitrile at 100℃; for 5h; | A 92% B n/a |
-
-
63294-84-8
(E)-6-pentadecen-15-olide

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium-barium carbonate under 1520 Torr; for 3h; Ambient temperature; | 99% |
-
-
76293-72-6, 76293-73-7, 4941-77-9
(E/Z)-oxacyclohexadec-11-en-2-one

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 16h; Solvent; Concentration; Schlenk technique; Glovebox; | 99% |
| With hydrogen; palladium on activated charcoal under 760 Torr; | 94% |
| With hydrogen; palladium on activated charcoal | 94% |
| With hydrogen; platinum(IV) oxide under 760 Torr; | |
| With 5%-palladium/activated carbon; hydrogen In methanol at 25℃; for 24h; | 89.2 %Chromat. |
-
-
87227-39-2
E-Pentadec-2-ene-15-olide

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal | 99% |
| With hydrogen; palladium on activated charcoal In ethyl acetate at 20℃; under 760 Torr; for 3h; | 98% |
| With hydrogen; palladium on activated charcoal In ethanol; ethyl acetate for 48h; Ambient temperature; | 48 mg |

| Conditions | Yield |
|---|---|
| With dmap; polymer bound carbodiimide; 4-(dimethylamino)pyridine hydrochloride In tetrahydrofuran; chloroform Cyclization; lactonisation; Heating; | 97% |
| With dmap; 4-(dimethylamino)pyridine hydrochloride; dicyclohexyl-carbodiimide In tetrahydrofuran; chloroform Heating; | 95% |
| With dmap; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In tetrahydrofuran; chloroform for 18h; Reflux; Inert atmosphere; | 95% |
-
-
195320-68-4
(Z)-Oxacyclohexadec-6-en-2-one

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal under 760 Torr; | 95% |
| With hydrogen; palladium on activated charcoal | 95% |
-
-
93472-40-3
trimethylsilyl 15-(trimethylsiloxy)pentadecanoate

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| dipropylboryl triflate In toluene Heating; | 94% |

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 70 - 250℃; under 3.75038 Torr; for 0.333333h; Reagent/catalyst; Inert atmosphere; | 92.9% |
-
-
118072-05-2
12-iodo-15-pentadecanolide

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene Irradiation; | 92% |
| With 2,2'-azobis(isobutyronitrile); iodine; tri-n-butyl-tin hydride; 1,8-diazabicyclo[5.4.0]undec-7-ene 1.) THF, reflux, 40 min, 2.) THF, ether; Multistep reaction; |

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Heating; | 92% |
| With potassium carbonate In acetone for 48h; Heating; Yield given; |

-
A
-
106-02-5
pentadecanolide

-
B
-
16697-83-9
N-methyl-N-tosylacetamide

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In dichloromethane at 20℃; for 4h; | A 92% B n/a |

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane at 20℃; | 92% |
-
-
77744-43-5
15-(tert-butyldimethylsilyloxy)-pentadecanoic acid

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In chloroform for 18h; Reflux; Inert atmosphere; | 91% |
| Multi-step reaction with 4 steps 1.1: n-BuLi / diethyl ether / 0 °C 1.2: (COCl)2; DMF / diethyl ether 2.1: Et3N / CH2Cl2 / 0 - 20 °C 3.1: 96 percent / 3HF*Et3N / acetonitrile / 20 °C 4.1: 58 percent / Cu(OTf)2 / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: n-BuLi / diethyl ether / 0 °C 1.2: (COCl)2; DMF / diethyl ether 2.1: Et3N / CH2Cl2 / 0 - 20 °C 3.1: 57 percent / 3HF*Et3N / acetonitrile / 20 °C 4.1: 83 percent / Cu(OTf)2 / tetrahydrofuran / 20 °C View Scheme |
-
-
38223-29-9
12-oxo-15-pentadecanolide

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In acetic anhydride at 0 - 5℃; for 1h; | 90% |
| With hydrogenchloride; zinc In toluene at 0℃; | 84% |
| With sodium cyanoborohydride; toluene-4-sulfonic acid; toluene-4-sulfonic acid hydrazide 1.) DMF, sulfolane, 15 min., 2.) cyclohexane, reflux, 4 h; | 50% |
| Multi-step reaction with 2 steps 1: methanol / 0.67 h / Heating 2: <<(C6H5)3P>Cu>BH4 / CHCl3 / 4 h / Heating View Scheme |
-
-
89611-22-3
15-Hydroxy-pentadecanoic acid (Z)-2-dimethylcarbamoyl-1-methyl-vinyl ester

-
A
-
106-02-5
pentadecanolide

-
B
-
2044-64-6
N,N-Dimethylacetoacetamid

| Conditions | Yield |
|---|---|
| With magnesium bromide In tetrahydrofuran at 55℃; other solvents, temperatures and reagent; | A 90% B n/a |
-
-
81238-39-3
13-Benzenesulfonyl-oxacyclohexadecan-2-one

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; sodium amalgam In methanol; 1,2-dimethoxyethane at -25℃; for 3h; | 90% |
-
-
109182-98-1
Acrylic acid 12-iodo-dodecyl ester

-
A
-
106-02-5
pentadecanolide

-
B
-
659-76-7
1,17-dioxa-cyclodotriacontane-2,18-dione

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In methanol for 3h; Ambient temperature; Irradiation; | A 90% B 7 % Chromat. |
-
-
93472-40-3
trimethylsilyl 15-(trimethylsiloxy)pentadecanoate

-
A
-
106-02-5
pentadecanolide

-
B
-
659-76-7
1,17-dioxa-cyclodotriacontane-2,18-dione

| Conditions | Yield |
|---|---|
| With 4-(trifluoromethyl)benzoic anhydride; silver perchlorate; titanium tetrachloride In dichloromethane for 3h; Ambient temperature; | A 89% B 4% |
| With 4-(trifluoromethyl)benzoic anhydride; silver perchlorate; titanium tetrachloride In dichloromethane; toluene at 20℃; for 33h; | A 89% B 2% |
-
-
173790-14-2
Z-oxacyclohexadec-3-en-2-one

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With samarium diiodide; 2,4-dichlorophenoxyacetic acid dimethylamine; tert-butyl alcohol In tetrahydrofuran for 0.5h; Ambient temperature; | 88% |
-
-
76529-42-5
methyl 15-hydroxypentadecanoate

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With Lipase B immobilized on acrylic resin from Candida antarctica In cyclohexane; water at 40℃; for 2h; Enzymatic reaction; | 88% |
| Multi-step reaction with 4 steps 1: Imidazole / dimethylformamide 2: 5.0 M NaOH / tetrahydrofuran; methanol; H2O / 7 h 3: 1.) DCC, DMAP; 2.) CH3COOH / 1.) THF, -25 deg C, 4 days; 2.) H2O, room temp., 8 h 4: 45 percent Chromat. / t-BuOK / benzene; tetrahydrofuran View Scheme | |
| Multi-step reaction with 4 steps 1: Imidazole / dimethylformamide 2: 5.0 M NaOH / tetrahydrofuran; methanol; H2O / 7 h 3: 1.) DCC, DMAP; 2.) CH3COOH / 1.) THF, -25 deg C, 4 days; 2.) H2O, room temp., 8 h 4: 74 percent / t-BuOK, molecular sieves / benzene; tetrahydrofuran / Heating; also without molecuar sieves and reflux View Scheme | |
| Multi-step reaction with 4 steps 1: Imidazole / dimethylformamide 2: 5.0 M NaOH / tetrahydrofuran; methanol; H2O / 7 h 3: 1.) 1-<3-(Dimethylamino)propyl>-3-ethylcarboximide hydrochloride, DMAP; 2.) CH3COOH / 1.) DMF, 1 day; 2.) H2O, 12 h 4: 81 percent / t-BuOK, molecular sieves / benzene; tetrahydrofuran / Heating; also without molecuar sieves and reflux View Scheme | |
| Multi-step reaction with 4 steps 1: Imidazole / dimethylformamide 2: 5.0 M NaOH / tetrahydrofuran; methanol; H2O / 7 h 3: 1.) DCC, DMAP; 2.) CH3COOH / 1.) THF, -25 deg C, 5 days; 2.) THF-H2O, room temp., 20 h 4: 43 percent Chromat. / t-BuOK, 19-crown-6 / benzene; tetrahydrofuran View Scheme |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 108h; | 86% |
| Stage #1: cyclopentadecanone With N-hydroxyphthalimide; 1,1-Diphenylmethanol; 2,2'-azobis(isobutyronitrile); oxygen In acetonitrile at 75℃; under 760.051 Torr; for 22h; Baeyer-Villiger oxidation; Stage #2: With 1,1,1,3',3',3'-hexafluoro-propanol; toluene-4-sulfonic acid at 60℃; Baeyer-Villiger oxidation; Inert atmosphere; | 70% |
| With potassium peroxomonosulphate; sulfuric acid; Petroleum ether at 50 - 65℃; |
-
-
56523-59-2
15-bromo-pentadecanoic acid

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With tetraoctylammonium (2-pyrrolidonide) In N,N-dimethyl-formamide for 24h; Ambient temperature; | 84% |
| With potassium carbonate In dimethyl sulfoxide at 75℃; for 4h; | 78% |
| With potassium carbonate; butanone |
-
-
4617-33-8
15-hydroxylpentadecanoic acid

-
-
14338-32-0
2-chloro-1-methyl-pyridinium iodide

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile | 84% |
| With tributyl-amine In acetonitrile | 74% |
| With triethylamine In toluene | 63%. |
-
-
874583-99-0
15-hydroxy-pentadecanoic acid 6-methyl-pyridin-2-yl ester

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) In tetrahydrofuran at 20℃; | 83% |
-
-
77744-39-9
15-Hydroxy-pentadecanethioic acid S-[(2R,3aR,19aS)-1-(tetradecahydro-4,7,10,13,16,19-hexaoxa-cyclopentacyclooctadecen-2-yl)methyl] ester

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| With molecular sieve; potassium tert-butylate In tetrahydrofuran; benzene Heating; | 81% |
| With molecular sieve; potassium tert-butylate In tetrahydrofuran; benzene Product distribution; Heating; also without molecuar sieves and reflux; | 81% |
-
-
122695-16-3
N-nitro-15-pentadecanelactam

-
-
106-02-5
pentadecanolide

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 12h; Heating; | 80% |
-
-
76529-42-5
methyl 15-hydroxypentadecanoate

-
A
-
106-02-5
pentadecanolide

-
B
-
659-76-7
1,17-dioxa-cyclodotriacontane-2,18-dione

| Conditions | Yield |
|---|---|
| In benzene at 40℃; for 72h; Lipase P; | A 78% B n/a |
| With Lipase P In benzene at 40℃; for 72h; Product distribution; |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile | 78% |

| Conditions | Yield |
|---|---|
| With sulfuric acid Reflux; | 100% |
| With sulfuric acid Reflux; | 100% |
| With toluene-4-sulfonic acid for 24h; Esterification; Ring cleavage; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With (Z)-9-octadecen-1-amine; lipase PS from Pseudomonas cepacia; water In Hexadecane at 40℃; for 0.5h; Miniemulsion system; Enzymatic reaction; | 100% |
| With sodium hydroxide In tetrahydrofuran; methanol; water | 100% |
| With potassium hydroxide In methanol; water for 5.5h; Heating; | 99% |
-
-
106-02-5
pentadecanolide

-
-
71736-22-6
15-iodo-pentadecanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide In acetic acid for 3h; Ring cleavage; iodination; Heating; | 100% |
| With aluminium(III) iodide In acetonitrile at 80℃; for 18h; | 99% |
| With hydrogen iodide In acetic acid for 6h; Heating; | 93% |
-
-
106-02-5
pentadecanolide

-
-
871570-58-0
potassium 15-hydroxypentadecanoate

| Conditions | Yield |
|---|---|
| With ethanol; potassium hydroxide for 2h; Reflux; | 100% |
| With potassium hydroxide In ethanol for 2h; Reflux; | 100% |
| With potassium hydroxide In ethanol for 2h; Reflux; | 100% |
| With potassium hydroxide In methanol |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 16h; Inert atmosphere; Darkness; | 99% |
| In methanol at 20℃; | 80% |
-
-
106-02-5
pentadecanolide

-
-
14722-40-8
1,15-pentadecanediol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 72h; | 98.6% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; | 97% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; | 96% |
-
-
106-02-5
pentadecanolide

-
-
56523-59-2
15-bromo-pentadecanoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; hydrogen bromide for 84h; Heating; | 98% |
| With hydrogen bromide; acetic acid at 60℃; for 16h; Product distribution / selectivity; Autoclave; Inert atmosphere; | 91% |
| With sulfuric acid; hydrogen bromide for 20h; Heating; | 84% |
-
-
67-56-1
methanol

-
-
106-02-5
pentadecanolide

-
-
124-41-4
sodium methylate

-
-
76529-42-5
methyl 15-hydroxypentadecanoate

| Conditions | Yield |
|---|---|
| for 3h; Heating / reflux; | 98% |


| Conditions | Yield |
|---|---|
| Stage #1: pentadecanolide; methylmagnesium bromide In tetrahydrofuran at -78 - 20℃; for 16h; Inert atmosphere; Stage #2: With acetic acid In tetrahydrofuran; water Cooling with ice; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 250℃; for 18h; Product distribution; autoclave, other base, other temperature; | 95% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 0.5h; | 91% |

| Conditions | Yield |
|---|---|
| With lipase PS from Pseudomonas cepacia; water In Hexadecane at 40℃; for 192h; Miniemulsion system; Enzymatic reaction; | 91% |
Cyclopentadecanolide Specification
The Cyclopentadecanolide, with the CAS registry number 106-02-5, is also known as 15-Hydroxypentadecanoic acid lactone. Its EINECS number is 203-354-6. This chemical's molecular formula is C15H28O2 and molecular weight is 240.38. What's more, its systematic name is Oxacyclohexadecan-2-one. This chemical should be sealed and stored in a cool and ventilated place. Moreover, it should be protected from oxides, light, heat and fire. It plays a role of fixing agent in the essence. It is suitable for eastern and fantasy type essences, such as potpourri, elecampane and ambre. When using it, you must avoid contact with eyes.
Physical properties of Cyclopentadecanolide are: (1)ACD/LogP: 5.166; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.17; (4)ACD/LogD (pH 7.4): 5.17; (5)ACD/BCF (pH 5.5): 4969.45; (6)ACD/BCF (pH 7.4): 4969.45; (7)ACD/KOC (pH 5.5): 15396.09; (8)ACD/KOC (pH 7.4): 15396.09; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.436; (14)Molar Refractivity: 70.912 cm3; (15)Molar Volume: 271.377 cm3; (16)Polarizability: 28.112×10-24cm3; (17)Surface Tension: 28.47 dyne/cm; (18)Density: 0.886 g/cm3; (19)Flash Point: 143.392 °C; (20)Enthalpy of Vaporization: 58.88 kJ/mol; (21)Boiling Point: 344.797 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 15-hydroxy-pentadecanoic acid at the ambient temperature. This reaction will need reagents N,N,N'-N'-tetramethylurea, oxalyl chloride, collidine and solvents acetonitrile, diethyl ether with the reaction time of 46 hours. The yield is about 90%.

Uses of Cyclopentadecanolide: it can be used to produce 15-iodo-pentadecanoic acid by heating. It will need reagent HI and solvent acetic acid with the reaction time of 3 hours. The yield is about 100%.
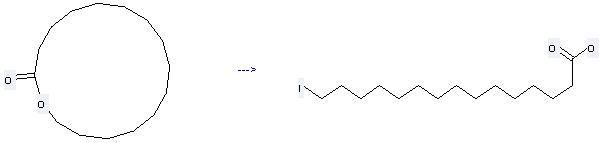
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1OCCCCCCCCCCCCCC1
(2)Std. InChI: InChI=1S/C15H28O2/c16-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-17-15/h1-14H2
(3)Std. InChIKey: FKUPPRZPSYCDRS-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 787, 1975. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 787, 1975. |
Related Products
- Cyclopentadecanolide
- 106032-61-5
- 10603-52-8
- 10603-92-6
- 106040-48-6
- 10604-21-4
- 10604-59-8
- 106047-29-4
- 10605-02-4
- 10605-09-1
- 10605-21-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View