-
Name
Methyl 3-nitrobenzoate
- EINECS 210-573-0
- CAS No. 618-95-1
- Article Data166
- CAS DataBase
- Density 1.301 g/cm3
- Solubility insoluble in water
- Melting Point 78-80 °C(lit.)
- Formula C8H7NO4
- Boiling Point 284.7 °C at 760 mmHg
- Molecular Weight 181.148
- Flash Point 136.8 °C
- Transport Information
- Appearance beige crystalline powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzoicacid, m-nitro-, methyl ester (6CI,7CI,8CI);3-Methoxycarbonyl-1-nitrobenzene;3-Nitrobenzoic acid methyl ester;Methyl 3-nitrobenzoate;Methylm-nitrobenzoate;NSC 1327;m-Carbomethoxynitrobenzene;m-Nitrobenzoic acidmethyl ester;
- PSA 72.12000
- LogP 1.90460
Synthetic route

| Conditions | Yield |
|---|---|
| With 1-(ferrocenylbutyl)-4-(3-methylimidazolium) tetrafluoroborate; iodine; potassium carbonate at 50 - 60℃; for 24h; Inert atmosphere; | 100% |
| With palladium 10% on activated carbon; oxygen; sodium carbonate at 90℃; under 15001.5 Torr; for 2h; Microwave irradiation; Green chemistry; | 98% |
| With 3-(7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methyl-1-(2,4,6-trimethylphenyl)-1H-imidazol-3-ium bis(trifluoromethanesulfonimide) salt; caesium carbonate In toluene at 60℃; for 3h; | 98% |

| Conditions | Yield |
|---|---|
| In 2-methyltetrahydrofuran; diethyl ether at 20℃; for 0.486667h; Flow reactor; | 99% |
| With diethyl ether | |
| In diethyl ether | |
| at 0℃; for 0.25h; | 62.3 mg |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; 2-chloro-4-methoxy-6-(N-phenylbenzamido)-1,3,5-triazine at 20℃; for 1h; | 99% |
| With oxone at 65℃; for 30h; | 98% |
| Stage #1: 3-nitrobenzoic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; Stage #2: methanol In dichloromethane for 0.0833333h; | 98% |
-
-
67-56-1
methanol

-
-
645-00-1
m-iodonitrobenzene

-
-
201230-82-2
carbon monoxide

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With triethylamine at 100℃; under 3750.38 Torr; for 1.5h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| With oxone at 65℃; for 30h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 2h; | 98% |
| In acetonitrile for 8h; Inert atmosphere; Alkaline conditions; |

| Conditions | Yield |
|---|---|
| With sodium carbonate; palladium; silver(l) oxide at 80℃; for 48h; Molecular sieve; | 98% |
| Stage #1: 3-Nitrobenzyl alcohol In toluene at 80℃; for 2h; Sonication; Stage #2: methanol With Oxone In toluene for 1.5h; Sonication; | 90% |
| With dibromamine-T; potassium carbonate In acetonitrile at 20℃; for 0.5h; Reagent/catalyst; Solvent; | 88% |

| Conditions | Yield |
|---|---|
| With oxone at 65℃; for 30h; | 98% |

| Conditions | Yield |
|---|---|
| With oxone at 65℃; for 30h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitrobenzoic acid With potassium carbonate In acetone for 0.5h; Stage #2: dimethyl sulfate In acetone at 20℃; for 2h; | 94.3% |

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride; ethylammonium nitrate at -5 - 20℃; for 2h; Inert atmosphere; regioselective reaction; | 94% |
| With sulfuric acid; guanidine nitrate at 0 - 5℃; for 3h; | 76% |
| With boron trifluoride; nitric acid |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide for 0.0166667h; microwave irradiation; | 94% |
| With layered double hydroxide - supported L-methionine at 180℃; for 6h; Autoclave; chemoselective reaction; | 89% |
-
-
121-92-6
3-nitrobenzoic acid

-
-
119-36-8
methyl salicylate

-
A
-
618-95-1
methyl 3-nitrobenzoate

-
B
-
69-72-7
salicylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitrobenzoic acid With potassium carbonate In N,N-dimethyl acetamide at 110℃; for 0.5h; Stage #2: methyl salicylate at 110℃; for 24h; | A 93% B n/a |

-
-
85107-55-7
(4-(methoxycarbonyl)-2-nitrophenyl)boronic acid

-
A
-
65235-35-0
dimethyl 2,2’-dinitro-[1,1’-biphenyl]-4,4’-dicarboxylate

-
B
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium fluoride dihydrate; tri tert-butylphosphoniumtetrafluoroborate In tetrahydrofuran for 1h; Suzuki-Miyaura Coupling; Inert atmosphere; Reflux; Schlenk technique; | A 10% B 88% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide dimethyl acetal for 16h; Heating; | 87% |
-
-
121-92-6
3-nitrobenzoic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| In methanol; toluene at 20℃; for 1.5h; | 87% |
| In methanol; toluene at 20℃; for 1.5h; | 86.79% |
-
-
93415-79-3
methyl 2-iodo-3-nitrobenzoate

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With air; tributyl borane; water In benzene at 20℃; | 85% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide dimethyl acetal for 16h; Heating; | 84% |
-
-
4731-65-1
tris(o-methoxyphenyl)phosphine

-
-
121-92-6
3-nitrobenzoic acid

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In cyclohexane at 120℃; for 12h; Schlenk technique; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| With 2-nitrobenzeneseleninic acid; dihydrogen peroxide at 20℃; for 48h; | 80% |
-
-
93415-79-3
methyl 2-iodo-3-nitrobenzoate

-
-
536-74-3
phenylacetylene

-
A
-
894854-29-6
methyl 3-nitro-2-(2-phenylethynyl)benzoate

-
B
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; diisopropylamine; bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 45℃; for 48h; Sonogashira coupling; | A 80% B 28% |


| Conditions | Yield |
|---|---|
| With pyridine; ferric(III) bromide; oxygen In chlorobenzene at 130℃; for 9h; Sealed tube; | 80% |

| Conditions | Yield |
|---|---|
| With 2-nitrobenzeneseleninic acid; dihydrogen peroxide at 65℃; for 48h; | 79% |
-
-
99769-19-4
[3-(methoxycarbonyl)phenyl]boronic acid

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate In toluene at 80℃; for 18h; Inert atmosphere; | 78% |
| Multi-step reaction with 2 steps 1.1: potassium hydrogen bifluoride / methanol; water / 0.03 h / 0 - 20 °C / Inert atmosphere 1.2: 0.02 h / 20 °C 2.1: Oxone / water; acetone / 2 h / 60 °C View Scheme |
-
-
108030-46-2
(E,E)-1-(N,N-dimethylamino)-4-nitro-1,3-butadiene

-
-
922-67-8
propynoic acid methyl ester

-
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| In xylene for 8h; Heating; | 74.8% |
-
-
67-56-1
methanol

-
-
99-61-6
3-nitro-benzaldehyde

-
A
-
618-95-1
methyl 3-nitrobenzoate

-
B
-
121-92-6
3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; calcium chloride at 65℃; for 48h; Reagent/catalyst; | A 74% B n/a |
| With dihydrogen peroxide at 20 - 70℃; for 15h; Green chemistry; | A 68% B 31% |
| With dihydrogen peroxide at 20℃; for 8h; |


| Conditions | Yield |
|---|---|
| Stage #1: propan-1-ol; 3-nitro-benzaldehyde With sodium hypochlorite; sodium iodide In water for 0.166667h; Cooling with ice; Stage #2: With sodium hypochlorite In water for 1.33333h; Cooling with ice; | 74% |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 80℃; for 2h; Schlenk technique; Inert atmosphere; | 74% |
| With potassium carbonate at 80℃; for 2h; Inert atmosphere; Sealed tube; | 74% |

| Conditions | Yield |
|---|---|
| With pyridine; ferric(III) bromide; oxygen In chlorobenzene at 130℃; for 9h; Sealed tube; | 74% |
-
-
67-56-1
methanol

-
-
420-04-2
CYANAMID

-
-
99-61-6
3-nitro-benzaldehyde

-
A
-
1196908-12-9
methyl N-cyano-3-nitrobenzimidate

-
B
-
618-95-1
methyl 3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; sodium t-butanolate at 50℃; for 12h; | A 72% B 16% |


| Conditions | Yield |
|---|---|
| With potassium fluoride; polymethylhydrosiloxane; palladium diacetate In tetrahydrofuran; water at 20℃; for 0.5h; | 100% |
| With potassium fluoride; polymethylhydrosiloxane; palladium diacetate In tetrahydrofuran at 20℃; for 0.5h; | 100% |
| With 5%-palladium/activated carbon; hydrogen In methanol at 20℃; | 100% |
-
-
618-95-1
methyl 3-nitrobenzoate

-
-
36475-13-5
methyl 3-(hydroxylamino)benzoate

| Conditions | Yield |
|---|---|
| With 5% Rh/C; hydrazine hydrate In tetrahydrofuran at 0℃; Inert atmosphere; Schlenk technique; Sealed tube; | 100% |
| With 5% rhodium-on-charcoal; hydrazine hydrate In tetrahydrofuran at 0℃; for 2.5h; Inert atmosphere; | 99% |
| With 5% rhodium-on-charcoal; hydrazine hydrate In tetrahydrofuran at 0℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With sulfur In methanol at 65℃; for 5h; Time; | 96.4% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol for 4h; Reflux; | 96% |
| With hydrazine hydrate In ethanol at 85℃; for 4h; | 96.2% |
| With hydrazine hydrate In ethanol for 0.2h; Reflux; Microwave irradiation; | 95% |
-
-
618-95-1
methyl 3-nitrobenzoate

-
-
824-79-3
sodium 4-methylbenzenesulfinate

-
-
173436-66-3
methyl 3-{[(4-methylphenyl)sulfonyl]amino}benzoate

| Conditions | Yield |
|---|---|
| With trans-N,N'-dimethyl-1,2-cyclohexyldiamine; sodium hydrogen sulfite; iron(II) chloride In dimethyl sulfoxide at 60℃; for 12h; Sealed tube; Inert atmosphere; | 95% |
| With sodium hydrogensulfite In water at 60℃; for 20h; chemoselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium hydroxide In methanol at 20℃; pH=> 10; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-nitrobenzoate With ammonia In ethylene glycol at 40 - 45℃; for 20h; Stage #2: sodium methylate In ethylene glycol at 40 - 45℃; for 5h; Conversion of starting material; | 92% |
| With ammonia; sodium methylate In methanol at 60 - 65℃; for 26h; Conversion of starting material; | 68% |
| Stage #1: methyl 3-nitrobenzoate With ammonia In butan-1-ol at 40 - 45℃; for 20h; Stage #2: sodium methylate In butan-1-ol at 40 - 45℃; for 8h; Conversion of starting material; |

| Conditions | Yield |
|---|---|
| With potassium phosphate; bis(acetylacetonato)palladium(II); dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine In cyclohexane at 130℃; for 24h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; ethanol; cerium(III) chloride heptahydrate at 20℃; for 24h; chemospecific reaction; | 91% |
| With sodium tetrahydroborate; zinc phthalocyanine In PEG-400 at 100℃; for 10h; | 90% |
| With sodium tetrahydroborate In 1,4-dioxane; water for 2h; Ambient temperature; | 80% |
-
-
17715-69-4
1-Bromo-2,4-dimethoxybenzene

-
-
618-95-1
methyl 3-nitrobenzoate

-
-
864745-95-9
2,2',4,4'-tetramethoxy-3''-nitrotrityl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromo-2,4-dimethoxybenzene With iodine; magnesium In tetrahydrofuran for 0.333333h; Stage #2: methyl 3-nitrobenzoate In tetrahydrofuran at -78℃; | 91% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; dibromoisocyanuric acid | 90% |
| With sulfuric acid; dibromoisocyanuric acid at 20℃; for 2h; | 86% |
| With sulfuric acid; dibromoisocyanuric acid at 20℃; for 2h; Inert atmosphere; Schlenk technique; | 86% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Cumene hydroperoxide In ammonia at -33℃; | 90% |
-
-
618-95-1
methyl 3-nitrobenzoate

-
-
156-87-6
propan-1-ol-3-amine

-
-
254454-76-7
N-(3-Hydroxypropyl)-3-nitrobenzamide

| Conditions | Yield |
|---|---|
| In toluene at 94℃; for 3.5h; Acylation; | 90% |
-
-
618-95-1
methyl 3-nitrobenzoate

-
-
75-65-0
tert-butyl alcohol

-
-
58656-99-8
3-nitro-benzoic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane for 1h; | 89% |
Methyl 3-nitrobenzoate Specification
The cas register number of Methyl 3-nitrobenzoate is 618-95-1. It also can be called as m-Nitrobenzoic acid, methyl ester and the IUPAC Name about this chemical is methyl 3-nitrobenzoate. It belongs to the following product categories, such as Aromatic Esters, C8 to C9, Carbonyl Compounds, Esters and so on. When you are using it, please avoid contact with skin and eyes.
Physical properties about Methyl 3-nitrobenzoate are: (1)ACD/LogP: 1.82; (2)ACD/LogD (pH 5.5): 1.82; (3)ACD/LogD (pH 7.4): 1.82; (4)ACD/BCF (pH 5.5): 14.2; (5)ACD/BCF (pH 7.4): 14.2; (6)ACD/KOC (pH 5.5): 232.46; (7)ACD/KOC (pH 7.4): 232.46; (8)#H bond acceptors: 5; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 72.12Å2; (11)Index of Refraction: 1.553; (12)Molar Refractivity: 44.57 cm3; (13)Molar Volume: 139.1 cm3; (14)Polarizability: 17.66x10-24cm3; (15)Surface Tension: 48.6 dyne/cm; (16)Enthalpy of Vaporization: 52.37 kJ/mol; (17)Boiling Point: 284.7 °C at 760 mmHg; (18)Vapour Pressure: 0.00293 mmHg at 25°C.
Preparation: this chemical can be prepared by methanol and 3-nitro-benzaldehyde oxime. This reaction will need reagent 2-nitrobenzeneseleninic acid (2-NBSeA), 30percent aq. H2O2. The reaction time is 2 day(s) with reaction temperature of 20 ℃. The yield is about 80%.
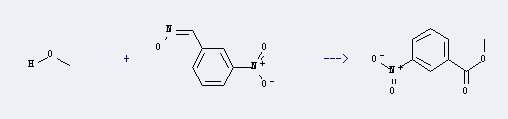
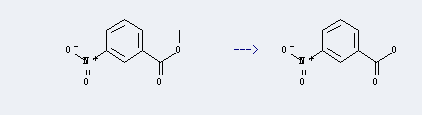
Uses of p-Chloropropiophenone: it can be used to produce 3-nitro-benzoic acid at temperature of 190 ℃. This reaction will need reagent PhSH, KF and solvent various solvent(s) with reaction time of 10 min. The yield is about 70%.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: COC(=O)C1=CC(=CC=C1)[N+](=O)[O-]
(2)InChI: InChI=1S/C8H7NO4/c1-13-8(10)6-3-2-4-7(5-6)9(11)12/h2-5H,1H3
(3)InChIKey: AXLYJLKKPUICKV-UHFFFAOYSA-N
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 618-98-4
- 61898-95-1
- 61901-15-3
- 61901-18-6
- 619-01-2
- 61901-21-1
- 61901-25-5
- 61901-27-7
- 61901-32-4
- 61901-34-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View