-
Name
5-Hydroxytryptamine
- EINECS 200-058-9
- CAS No. 50-67-9
- Article Data43
- CAS DataBase
- Density 1.288 g/cm3
- Solubility 0.5 g/100 mL (25 oC)
- Melting Point 22-23°C(lit.)
- Formula C10H12N2O
- Boiling Point 416.089 °C at 760 mmHg
- Molecular Weight 176.218
- Flash Point 205.443 °C
- Transport Information
- Appearance White powder
- Safety 36/37-45
- Risk Codes 63-25
-
Molecular Structure
- Hazard Symbols T
- Synonyms Indol-5-ol,3-(2-aminoethyl)- (6CI,8CI);3-(2-Aminoethyl)indol-5-ol;3-(b-Aminoethyl)-5-hydroxyindole;5-Hydroxy-3-(b-aminoethyl)indole;Tryptamine, 5-hydroxy-;5-Hydroxytryptamine;
- PSA 62.04000
- LogP 2.07500
Synthetic route
-
-
147918-24-9
N-Benzyl-2-<5-(benzyloxy)-3-indolyl>-1-ethanamin

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; ammonium formate In methanol at 70℃; for 0.75h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; aromatic L-amino acid decarboxylase In various solvent(s) at 30℃; for 48h; | 97% |
| With NH4OH-NH4Cl buffer; pyridoxal 5'-phosphate at 30℃; for 0.5h; relative rate of CO2 evolution by aromatic L-amino acid decarboxylase from Micrococcus percitreus; | |
| With isopropyl β-D-thiogalactoside; L-tryptophan decarboxylase in recombinant Escherichia coli at 28℃; |
-
-
77549-09-8
5-hydroxy-Nb-methoxycarbonyltryptamine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Heating; | 73% |
| With sodium hydroxide In methanol for 4h; Heating; | 73% |

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 20℃; | 65% |
| With aluminium trichloride; benzene |
-
-
20776-45-8
5-benzyloxytryptamine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With methanol; palladium on activated charcoal Hydrogenation; | |
| With palladium on activated charcoal; ethanol; water Hydrogenation; | |
| With methanol; Pd-BaSO4 Hydrogenation; |
-
-
93331-75-0
5-benzyloxy-3-(2-nitro-ethyl)-indole

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With platinum Hydrogenation; | |
| With palladium on activated charcoal Hydrogenation; |
-
-
55895-70-0
[2-(5-benzyloxy-indol-3-yl)-ethyl]-carbamic acid benzyl ester

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium on activated charcoal; ethanol Hydrogenation.und Wasser; |

| Conditions | Yield |
|---|---|
| biotransformation by cell cultures of Peganum harmala; | |
| With CYP71P1; NADPH; NADPH-P450 reductase at 30℃; for 0.166667h; pH=7.25; Kinetics; aq. phosphate buffer; Enzymatic reaction; |
-
-
343-94-2
tryptamine hydochloride

-
A
-
15700-23-9
7-Hydroxytryptamine

-
B
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
C
-
443-31-2
6-hydroxytryptamine

-
D
-
570-14-9
4-Hydroxytryptamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide; ascorbic acid 1.) H2O, 2.) phosphate buffer (pH= 7.2, 0.1 M), 22 deg C, 2.5 min; Multistep reaction. Title compound not separated from byproducts; |

-
-
53157-50-9
N-Cbz-5-hydroxytryptamine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol Yield given; |

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate mammalian aromatic L-amino acid decarboxylase; | |
| With Papaver somniferum tyrosine decarboxylase S372G mutant In aq. phosphate buffer at 25℃; pH=7.5; Kinetics; Reagent/catalyst; Enzymatic reaction; |

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With water; sodium hydrogencarbonate; potassium hexacyanoferrate(III) |
-
-
40619-73-6
2-(1-benzyl-5-methoxy-1H-indol-3-yl)ethylamine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 66 percent / Na / tetrahydrofuran; liquid ammonia / 1.5 h / -33 °C 2: 65 percent / BBr3 / CH2Cl2 / 20 °C View Scheme |
-
-
93879-42-6
1-benzyl-5-methoxy-1H-indole-3-acetamide

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 60.5 percent / LiAlH4 / diethyl ether / 48 h / Heating 2: 66 percent / Na / tetrahydrofuran; liquid ammonia / 1.5 h / -33 °C 3: 65 percent / BBr3 / CH2Cl2 / 20 °C View Scheme |
-
-
419569-93-0
methyl 1-benzyl-5-methoxy-1H-indole-3-acetate

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 83 percent / NH3 / methanol / 192 h / 20 °C 2: 60.5 percent / LiAlH4 / diethyl ether / 48 h / Heating 3: 66 percent / Na / tetrahydrofuran; liquid ammonia / 1.5 h / -33 °C 4: 65 percent / BBr3 / CH2Cl2 / 20 °C View Scheme |
-
-
185987-04-6
1-formyl-5-hydroxy-N-methoxycarbonyltryptamine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 76 percent / 2N NaOH / methanol 2: 73 percent / 10percent NaOH / methanol / Heating View Scheme |
-
-
38750-13-9
(2-indol-3-yl-ethyl)-carbamic acid benzyl ester

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) Pb(OAc)4; 2.) Zn powder / 1.) CF3COOH, CH2Cl2 2: H2 / 5percent Pd-C / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: acetic acid; dioxane 2: NaCN; aqueous ethanol 3: LiAlH4; diethyl ether 4: palladium/charcoal; ethanol / Hydrogenation View Scheme | |
| Multi-step reaction with 4 steps 1: phosphoryl chloride / und anschliessenden Hydrolysieren mit wss.Natronlauge bzw. wss.Natriumcarbonat-Loesung 2: ammonium acetate 3: LiAlH4; diethyl ether 4: methanol; palladium/charcoal / Hydrogenation View Scheme | |
| Multi-step reaction with 4 steps 1: phosphoryl chloride / und anschliessenden Hydrolysieren mit wss.Natronlauge bzw. wss.Natriumcarbonat-Loesung 2: benzylamine 3: LiAlH4; THF 4: palladium/charcoal; ethanol; water / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LiAlH4; diethyl ether 2: palladium/charcoal; ethanol / Hydrogenation View Scheme |
-
-
92438-11-4
5-Benzyloxy-2,ω-dinitro-styrol

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: ethanol; acetic acid 2: acetic acid; dioxane 3: NaCN; aqueous ethanol 4: LiAlH4; diethyl ether 5: palladium/charcoal; ethanol / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaCN; aqueous ethanol 2: LiAlH4; diethyl ether 3: palladium/charcoal; ethanol / Hydrogenation View Scheme | |
| Multi-step reaction with 3 steps 1: NaCN; aqueous ethanol 2: LiAlH4; diethyl ether 3: platinum/charcoal; methanol / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LiAlH4; diethyl ether 2: palladium/charcoal; ethanol / Hydrogenation View Scheme | |
| Multi-step reaction with 2 steps 1: LiAlH4; diethyl ether 2: platinum/charcoal; methanol / Hydrogenation View Scheme |
-
-
22424-62-0
(5-benzyloxy-indol-3-yl)-glyoxylic acid amide

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LiAlH4; diethyl ether; THF 2: palladium/BaSO4; methanol / Hydrogenation View Scheme |
-
-
22424-61-9
2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous NH3 <28percent > 2: LiAlH4; diethyl ether; THF 3: palladium/BaSO4; methanol / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: methanol. HCl 2: ethanol; N2H4+H2O 3: benzene; NaNO2; aqueous acetic acid / Erwaermen des Reaktionsprodukts in Benzol mit Benzylalkohol 4: palladium/charcoal; HCl; ethanol / Hydrogenation.und Wasser View Scheme |
-
-
101890-43-1
3-(5-benzyloxy-indol-3-yl)-propionic acid methyl ester

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethanol; N2H4+H2O 2: benzene; NaNO2; aqueous acetic acid / Erwaermen des Reaktionsprodukts in Benzol mit Benzylalkohol 3: palladium/charcoal; HCl; ethanol / Hydrogenation.und Wasser View Scheme |
-
-
101783-03-3
3-(5-benzyloxy-indol-3-yl)-propionic acid hydrazide

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: benzene; NaNO2; aqueous acetic acid / Erwaermen des Reaktionsprodukts in Benzol mit Benzylalkohol 2: palladium/charcoal; HCl; ethanol / Hydrogenation.und Wasser View Scheme |
-
-
7358-97-6
5-benzyloxy-3-(2-carboxy-ethyl)-indole-2-carboxylic acid

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: tetralin 2: methanol. HCl 3: ethanol; N2H4+H2O 4: benzene; NaNO2; aqueous acetic acid / Erwaermen des Reaktionsprodukts in Benzol mit Benzylalkohol 5: palladium/charcoal; HCl; ethanol / Hydrogenation.und Wasser View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonium acetate 2: LiAlH4; diethyl ether 3: methanol; palladium/charcoal / Hydrogenation View Scheme | |
| Multi-step reaction with 3 steps 1: benzylamine 2: LiAlH4; THF 3: palladium/charcoal; ethanol; water / Hydrogenation View Scheme |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
68573-23-9
(2E)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-3-(4-hydroxy-3-methoxyphenyl)-2-propenamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide | 99% |
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol With triethylamine In dichloromethane; N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 57% |
| Stage #1: 3-(2-aminoethyl)-1H-indol-5-ol; (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With pyridine; dicyclohexyl-carbodiimide at 20℃; for 24h; Stage #2: With potassium hydroxide In methanol at 20℃; for 4h; |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
433-27-2
Trifluoroacetaldehyde ethyl hemiacetal

-
-
126260-67-1
6-hydroxy-1-trifluoromethyl-1,2,3,4-tetrahydro-9H-pyrido<3,4-b>indole

| Conditions | Yield |
|---|---|
| at 110 - 120℃; for 5h; | 98.8% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
79-22-1
methyl chloroformate

-
-
77549-09-8
5-hydroxy-Nb-methoxycarbonyltryptamine

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 94% |
-
-
195299-77-5
(2R,4R)-2-[2-[4-fluoro-2-[2-(4-fluoro-3-methoxyphenyl)ethyl]phenoxy]ethyl]-4-hydroxy-1-methylpyrrolidine

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
645-66-9
lauric anhydride

| Conditions | Yield |
|---|---|
| With pyridine; dmap | 91% |

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
5801-57-0, 52178-60-6
(Z)-2-hydroxy-3-phenyl-acrylic acid

-
-
17994-22-8
1-benzyl-1,2,3,4-tetrahydro-8-hydroxy-β-carboline-1-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol for 48h; | 90% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
75-99-0
2,2-Dichloropropionic acid

-
-
1388185-92-9
C13H14Cl2N2O2

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 24h; | 89% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
506-30-9
Arachidic acid

-
-
21249-34-3
N-(2-(5-hydroxy-1H-indol-3-yl)ethyl)icosanamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine In N,N-dimethyl-formamide at 20℃; Reagent/catalyst; Solvent; | 88% |
-
-
23911-26-4
diethylenetriaminepentaacetic dianhydride

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
875429-83-7
bis-5-hydroxytriptamide-DPTA

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 48h; | 87% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 48h; | 58% |
| Stage #1: diethylenetriaminepentaacetic dianhydride; 3-(2-aminoethyl)-1H-indol-5-ol With pyridine; ascorbic acid In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With water |
-
-
108-05-4
vinyl acetate

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1210-83-9
N-acetyl-5-hydroxytryptamine

| Conditions | Yield |
|---|---|
| With agarose immobilized acetyltransferase from Mycobacterium smegmatis (MsAcT) In aq. phosphate buffer; dimethyl sulfoxide at 25℃; under 760.051 Torr; for 0.0833333h; pH=8.0; Flow reactor; Enzymatic reaction; chemoselective reaction; | 85% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
501-53-1
benzyl chloroformate

-
-
53157-50-9
N-Cbz-5-hydroxytryptamine

| Conditions | Yield |
|---|---|
| With sodium carbonate In water for 6h; Ambient temperature; | 84% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
66753-05-7
<5,6,8,9,11,12,14,15-3H8>arachidonic acid

| Conditions | Yield |
|---|---|
| Stage #1: <5,6,8,9,11,12,14,15-3H8>arachidonic acid With triethylamine; isobutyl chloroformate In acetonitrile at 23℃; for 2h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol In N,N-dimethyl-formamide at 23℃; for 20h; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: p-Coumaric Acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol With triethylamine In dichloromethane; N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 82% |
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide | 79% |
| Stage #1: 3-(2-aminoethyl)-1H-indol-5-ol; p-Coumaric Acid With pyridine; dicyclohexyl-carbodiimide at 20℃; for 24h; Stage #2: With potassium hydroxide In methanol at 20℃; for 4h; | 0.14 mmol |
-
-
112-77-6
(Z)-9-octadecenoyl chloride

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1002100-44-8
(Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-9-octadecenamide

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 5h; Cooling with ice; | 82% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
119768-45-5
(S)-2-((tert-butoxycarbonyl)amino)-3-(5-hydroxy-1H-indol-3-yl)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-2-((tert-butoxycarbonyl)amino)-3-(5-hydroxy-1H-indol-3-yl)propanoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 0.166667h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol In N,N-dimethyl-formamide for 2h; | 82% |
| Stage #1: (S)-2-((tert-butoxycarbonyl)amino)-3-(5-hydroxy-1H-indol-3-yl)propanoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol In N,N-dimethyl-formamide at 20℃; for 2h; | 390 mg |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 8h; | 75% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 8h; | 75% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 8h; | 75% |

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 5h; Cooling with ice; | 70% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
153821-44-4
2,5,7,8-tetramethyl-6-(oxiran-2-ylmethoxy)-2-(4,8,12-trimethyltridecyl)chroman

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-aminoethyl)-1H-indol-5-ol at -10℃; for 1h; Alkaline conditions; Stage #2: 2,5,7,8-tetramethyl-6-(oxiran-2-ylmethoxy)-2-(4,8,12-trimethyltridecyl)chroman In dichloromethane at -10℃; for 12h; regioselective reaction; | 70% |
-
-
470-17-7
isoalantolactone

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1353868-49-1
(3aR,8aR,9aR)-3-{[2-(5-hydroxy-1H-indol-3-yl)ethylamino]methyl}-8a-methyl-5-methylidene-decahydronaphtho[2,3-b]furan-2-one hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 20℃; Michael type reaction; stereospecific reaction; | 68% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
322474-21-5
N,N'-bis-Boc-S-methyl-isothiourea

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 67% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; | 65% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1448991-74-9
C18H16O7

-
-
1355043-50-3
Dimethyl-3-(2-hydroxybenzoyl)-9-hydroxy-6,7,12,12b-tetrahydroindolo[2,3-a]quinolizine-1,12b-dicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-aminoethyl)-1H-indol-5-ol; C18H16O7 With trimethyl orthoformate In dichloromethane at 20℃; Inert atmosphere; Stage #2: With trifluoroacetic acid In dichloromethane at 20℃; Inert atmosphere; | 64% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
7669-54-7
2-nitrobenzenesulfenyl chloride

-
-
102250-06-6
3-(2-Amino-ethyl)-2-(2-nitro-phenylsulfanyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| In acetic acid at 0℃; for 3h; | 63% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
291519-12-5
1-acetyl-2-methylsulfanyl-4-imidazolidinone

| Conditions | Yield |
|---|---|
| In ethanol for 96h; Reflux; | 60% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dichloro-3-(6-methyl-2-oxo-2H-chromen-4-yl)propanoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide for 0.333333h; Inert atmosphere; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol In N,N-dimethyl-formamide at 20℃; for 18h; Inert atmosphere; | 60% |
-
-
949890-75-9
(-)-(S)-3-(benzyloxycarbonylamino-methyl)-5-methyl-hexanoic acid

-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1224700-50-8
C26H33N3O4

| Conditions | Yield |
|---|---|
| Stage #1: (-)-(S)-3-(benzyloxycarbonylamino-methyl)-5-methyl-hexanoic acid With N-ethylmorpholine;; isobutyl chloroformate In tetrahydrofuran at -15℃; for 0.0333333h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol In tetrahydrofuran; water at -15 - 20℃; for 24.1667h; | 56% |
Serotonin History
Serotonin as originally discovered by Italian Vittorio Erspamer in Rome in 1935 and American scientists in the late 1940s. In 1948, it was isolated and named by Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic. It was initially identified as a vasoconstrictor substance in blood serum – hence serotonin, a serum agent affecting vascular tone. Serotonin was later chemically identified as 5-hydroxytryptamine (5-HT) by Rapport, and, as the broad range of physiological roles were elucidated, 5-HT became the preferred name in the pharmacological field.
Serotonin Specification
The Serotonin, with the CAS registry number 50-67-9, is also known as 5-Hydroxytryptamine. It belongs to the product categories of Tryptamines; Standards - 13C & 2H for GC-Mass Spectrometry. Its EINECS number is 200-058-9. This chemical's molecular formula is C10H12N2O and molecular weight is 176.21. What's more, its systematic name is 3-(2-Aminoethyl)-1H-indol-5-ol. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Neurotransmitter Agents; (3)Reproductive Effect; (4)Serotonin Agents; (5)Serotonin Receptor Agonists. This chemical is a monoamine neurotransmitter. It is used as a boiler water softener, degreasing detergent, metal corrosion inhibitor or anti-rust agent.
Physical properties of Serotonin are: (1)ACD/LogP: 0.545; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.53; (4)ACD/LogD (pH 7.4): -1.74; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 3; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 62.04 Å2; (13)Index of Refraction: 1.711; (14)Molar Refractivity: 53.495 cm3; (15)Molar Volume: 136.796 cm3; (16)Polarizability: 21.207×10-24cm3; (17)Surface Tension: 67.0 dyne/cm; (18)Density: 1.288 g/cm3; (19)Flash Point: 205.443 °C; (20)Enthalpy of Vaporization: 69.524 kJ/mol; (21)Boiling Point: 416.089 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 2-(5-methoxy-indol-3-yl)-ethylamine at the temperature of 20 °C. This reaction will need reagent BBr3 and solvent CH2Cl2. The yield is about 65%.
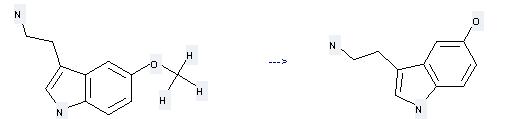
Uses of Serotonin: it can be used to produce N-Benzyloxycarbonyl-5-hydroxytryptamin at the ambient temperature. It will need reagent Na2CO3 and solvent H2O with the reaction time of 6 hours. The yield is about 84%.
.jpeg)
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc2c(cc1O)c(c[nH]2)CCN
(2)Std. InChI: InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2
(3)Std. InChIKey: QZAYGJVTTNCVMB-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 5mg/kg (5mg/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Farmakologiya i Toksikologiya Vol. 26, Pg. 10, 1963. |
| guinea pig | LD50 | intravenous | 12800ug/kg (12.8mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 168, Pg. 373, 1967. | |
| mouse | LD50 | intramuscular | 750mg/kg (750mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 112, Pg. 319, 1957. | |
| mouse | LD50 | intraperitoneal | 160mg/kg (160mg/kg) | Indian Journal of Physiology and Pharmacology. Vol. 17, Pg. 31, 1973. | |
| mouse | LD50 | intravenous | 81mg/kg (81mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Farmakologiya i Toksikologiya Vol. 26, Pg. 10, 1963. |
| mouse | LD50 | oral | 60mg/kg (60mg/kg) | Meditsinskii Zhurnal Uzbekistana. Vol. (3), Pg. 61, 1985. | |
| mouse | LD50 | subcutaneous | 601mg/kg (601mg/kg) | BEHAVIORAL: ATAXIA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Farmakologiya i Toksikologiya Vol. 26, Pg. 10, 1963. |
| mouse | LD50 | unreported | 250mg/kg (250mg/kg) | "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980Vol. 8(1), Pg. 122, 1982. | |
| rat | LD50 | intravenous | 30mg/kg (30mg/kg) | Psychopharmacology Service Center, Bulletin. Vol. 2, Pg. 17, 1963. | |
| rat | LD50 | subcutaneous | 285mg/kg (285mg/kg) | Psychopharmacology Service Center, Bulletin. Vol. 2, Pg. 17, 1963. |
Related Products
- Serotonin
- Serotonin adipinate
- Serotonin hydrochloride
- 5067-90-3
- 50679-08-8
- 5067-93-6
- 50-68-0
- 50681-25-9
- 50681-37-3
- 5068-28-0
- 5068-29-1
- 506-83-2
- 50685-26-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View