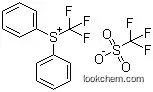
147531-11-1Relevant articles and documents
New Electrophilic Trifluoromethylating Agents
Yang, Jing-Jing,Kirchmeier, Robert L.,Shreeve, Jean'ne M.
, p. 2656 - 2660 (1998)
Synthetic routes to S-(trifluoromethyl)phenyl-4-fluorophenylsulfonium triflate (8), S-(trifluoromethyl)phenyl-2,4-difluorophenylsulfonium triflate (9), S-(trifluoromethyl)phenyl-3-nitrophenylsulfonium triflate (10), and S-(trifluoromethyl)-4-fluorophenyl-3-nitrophenylsulfonium triflate (11) are described. They are stable molecules and conveniently prepared by treating phenyl trifluoromethyl sulfoxide with benzene and its derivatives. These novel electrophilic trifluoromethylating agents react under mild conditions with a variety of aromatic rings (p-hydroquinone, pyrrole, and aniline) to give trifluoromethylated compounds (2-trifluoromethyl-p-hydroquinone, 2-trifluoromethylpyrrole, 2-trifluoromethylaniline, and 4-trifluoromethylaniline) in moderate to high yields. The electrophilic trifluoromethylating potential can be altered by placing electron-withdrawing substituents on the benzene rings.
Visible-Light Photocatalytic Tri- A nd Difluoroalkylation Cyclizations: Access to a Series of Indole[2,1- A[isoquinoline Derivatives in Continuous Flow
Yuan, Xin,Duan, Xiu,Cui, Yu-Sheng,Sun, Qi,Qin, Long-Zhou,Zhang, Xin-Peng,Liu, Jie,Wu, Meng-Yu,Qiu, Jiang-Kai,Guo, Kai
supporting information, p. 1950 - 1954 (2021/04/05)
A process for achieving photocatalyzed tri- A nd difluoromethylation/cyclizations for constructing a series of tri-or difluoromethylated indole[2,1-a]isoquinoline derivatives is described. This protocol utilized an inexpensive organic photoredox catalyst and provided good yields. Moreover, the combination of continuous flow and photochemistry, designed to provide researchers with a unique green process, was also shown to be key to allowing the reaction to proceed (product yield of 83% in flow vs 0% in batch).
Palladium-Catalyzed Arylation of Arylboronic Acids with Yagupolskii-Umemoto Reagents
Wang, Shi-Meng,Wang, Xiao-Yan,Qin, Hua-Li,Zhang, Cheng-Pan
supporting information, p. 6542 - 6546 (2016/05/02)
A Pd-catalyzed Suzuki cross-coupling of arylboronic acids with Yagupolskii-Umemoto reagents was explored. In contrary to trifluoromethylations, the Pd-catalyzed reaction of R-B(OH)2 and [Ar2SCF3]+[OTf]- provided the arylation products (R-Ar) in good to high yields. The reaction confirms that the S-Ar bonds of [Ar2SCF3]+[OTf]- can be readily cleaved in the presence of Pd complexes. The relatively electron-poor aryl groups of asymmetric [Ar1Ar2SCF3]+[OTf]- salts are more favorably transferred compared to the electron-rich ones. This reaction represents the first report of utilization of [Ar2SCF3]+[OTf]- as arylation reagents in organic synthesis.
Photoredox-Catalyzed Stereoselective Conversion of Alkynes into Tetrasubstituted Trifluoromethylated Alkenes
Tomita, Ren,Koike, Takashi,Akita, Munetaka
, p. 12923 - 12927 (2015/11/02)
A regio- and stereoselective synthesis of trifluoromethylated alkenes bearing four different substituents has been developed. Stereocontrolled sulfonyloxytrifluoromethylation of unsymmetric internal alkynes with an electrophilic CF3 reagent, namely the triflate salt of the Yagupol'skii-Umemoto reagent, in the presence of an Ir photoredox catalyst under visible-light irradiation afforded trifluoromethylalkenyl triflates with well-predictable stereochemistry resulting from anti addition of the trifluoromethyl and triflate groups. Subsequent palladium-catalyzed cross-couplings led to tetrasubstituted trifluoromethylated alkenes in a highly stereoselective manner. The present method is the first example of a facile one-pot synthesis of tetrasubstituted trifluoromethylated alkenes from simple alkynes.