Manufacture 6-(TRIFLUOROMETHYL)PYRIDINE-3-CARBOXALDEHYDE
Min.Order / FOB Price:Get Latest Price
| 0 Metric Ton |
Negotiable |
- Min.Order :0 Metric Ton
- Purity: >98%
Keywords
Ethyl chlorodifluoroacetate Ethyl chlorodifluoroacetate Good Supplier In China Ethyl chlorodifluoroacetate in dye
Quick Details
- Appearance:colorless transparent liquid
- Application:
- PackAge:50-200kg/drum
- ProductionCapacity:|Metric Ton|Day
- Storage:store in a cool,dry. Well ventilated place
- Transportation:flammable liquid, class: 3; UN3272, packing group: II
Superiority:
we have a land of 3 hectares and an experienced R&D team.
We establish closely cooperative relationships with Zhejiang University, Zhejiang University of Science and
Technology, Emden-Leer of Germany, etc.
Company Name: Nantong Baokai Chemical Co., Ltd
Year Established: 2006
Main products: fluoro-pharmaceutical intermediates
Details:
1. Introduction of Ethyl chlorodifluoroacetate (CAS No. 383-62-0)
Our Ethyl chlorodifluoroacetate products are mainly Ethyl chlorodifluoroacetate >98%. For being one kind of clear colorless liquid, this chemical could be stored in a cool, dry, well ventilated place. And its package information is 50-200kg/drum, with the transportation of flammable liquid, class: 3; UN3272. As to the appliation of Ethyl chlorodifluoroacetate, it could be applied in the production of pesticide, medicine, dyestuff, phosphate and flame retardant.
Our Certificate Of Analysis (COA) for Ethyl chlorodifluoroacetate >98% is as below:
| Test Item | Sandard | Test Result |
| Batch No. | - | 20110416 |
| Apprearance | Colorless Liquid | Colorless Liquid |
| Purity | ≥98.0% | 98.57% |
| Water | ≤0.1% | 0.046% |
| Weight (Kg) | - |
1 |
Ethyl chlorodifluoroacetate belongs to the Product Categories which include Pharmaceutical Intermediates; Fluorinated Building Blocks; Fluorinating Reagents & Building Blocks for Fluorinated Biochemical Compounds; Synthetic Organic Chemistry; C2 to C5; Carbonyl Compounds; Esters. For being stable under recommended storage conditions, Ethyl chlorodifluoroacetate should be avoided away from heat, flames, extremes of temperature and direct sunlight. And this chemical is incompatible with strong oxidizing agents, strong bases. Besides, its hazardous decomposition products formed under fire conditions are including Carbon oxides, Hydrogen chloride gas.
2. Properties of Ethyl chlorodifluoroacetate (CAS No. 383-62-0)
Physical properties of Ethyl chlorodifluoroacetate are as below: (1)ACD/LogP: 1.555; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.56; (4)ACD/LogD (pH 7.4): 1.56; (5)ACD/BCF (pH 5.5): 8.95; (6)ACD/BCF (pH 7.4): 8.95; (7)ACD/KOC (pH 5.5): 167.13; (8)ACD/KOC (pH 7.4): 167.13; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 26.3; (13)Index of Refraction: 1.374; (14)Molar Refractivity: 27.498 cm3; (15)Molar Volume: 120.373 cm3; (16)Polarizability: 10.901×10-24 cm3; (17)Surface Tension: 24.7830009460449 dyne/cm; (18)Density: 1.317 g/cm3; (19)Flash Point: 28.895 °C; (20)Enthalpy of Vaporization: 33.352 kJ/mol; (21)Boiling Point: 93.783 °C at 760 mmHg; (22)Vapour Pressure: 48.757999420166 mmHg at 25°C.
3. Structure Descriptors of Ethyl chlorodifluoroacetate (CAS No. 383-62-0)
You could convert the following datas into the molecular structure:
InChI:InChI=1S/C4H5ClF2O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChIKey:InChIKey=GVCAWQUJCHZRCB-UHFFFAOYSA-N
Smiles:O=C(OCC)C(F)(F)Cl
4. Safety Information of Ethyl chlorodifluoroacetate (CAS No. 383-62-0)
Hazard Codes F,C,Xi
RIDADR UN 2924 3/PG 2
WGK Germany 3
Hazard Note Flammable
HazardClass 3
PackingGroup II
(1). Highly flammable: This chemcial may catch fire in contact with air, only need brief contact with an ignition source, have a very low flash point or evolve highly flammable gases in contact with water. And it could causes burns.
(2). Corrosive: This chemcial may destroy living tissue on contact. And it is irritating to respiratory system.
(3). Irritant. For being irritating to eyes and respiratory system, Ethene,1,2-dichloro-1,2-difluoro- may cause inflammation to the skin or other mucous membranes, and it may toxic by inhalation.
Therefore, you should take the following measure to prevent from danger.
(1). Keep away from sources of ignition.
(2). In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
(3). Take off immediately all contaminated clothing.
(4). Wear suitable protective clothing, gloves and eye/face protection.
(5). In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
5. Use of Ethyl chlorodifluoroacetate (CAS No. 383-62-0)
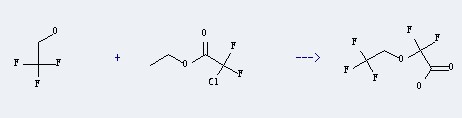
Ethyl chlorodifluoroacetate could react with 2,2,2-trifluoro-ethanol to produce (2,2,2-Trifluoroethoxy)difluoroacetic acid. This reaction could happen in the following condition: reagent of aq. KOH; reaction time of 40 hour(s); yield of 40%. Other condition is heating.
You Might Also Like
Related Searches
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View