- Min.Order :100 Gram
- Purity: 99%
- Payment Terms : L/C,T/T
Keywords
Palladium chloride PALLADIUM(+2)CHLORIDE 7647101 Cl2Pd PALLADIUM DICHLORIDE;PALLADIUM CHLORIDE(II)
Quick Details
- Appearance:Red-brown powder or dark brown powder
- Application:(1)used as the analysis reagents, such as determination of trace palladium, mercury, thallium, iodine, etc. (2) palladium test strips is used to test carbon monoxide. (3) also used to search for cra
- PackAge:as customer’s requirement.
- ProductionCapacity:600|Kilogram|Month
- Storage:room temperature
- Transportation:BY AIR/ BY SEA
Superiority:
| Palladium chloride Basic information |
| Physical and chemical properties Palladium Precious metal catalyst Production methods |
| Product Name: | Palladium chloride |
| Synonyms: | PALLADIUM(+2)CHLORIDE;PALLADIUM DICHLORIDE;PALLADIUM CHLORIDE(II);PALLADIUM CHLORIDE;PALLADIUM(II) CHLORIDE;enplateactivator440;nci-c60184;palladiumchloride(pdcl2) |
| CAS: | 7647-10-1 |
| MF: | Cl2Pd |
| MW: | 177.33 |
| EINECS: | 231-596-2 |
| Product Categories: | blocks;Inorganics;Catalysts for Organic Synthesis;Classes of Metal Compounds;Homogeneous Catalysts;Pd (Palladium) Compounds;Synthetic Organic Chemistry;Titanium Alkoxides, etc. (Homogeneous Catalysts);Transition Metal Compounds;Homogeneous Pd CatalystsMetal and Ceramic Science;Palladium Salts;Salts;Catalysis and Inorganic Chemistry;Homogeneous Pd Catalysts;Palladium;Crystal Grade Inorganics;PalladiumMetal and Ceramic Science;metal halide;chemical reaction,pharm,electronic,materials |
| Mol File: | 7647-10-1.mol |
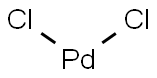 |
|
| Palladium chloride Chemical Properties |
| mp | 678-680 °C(lit.) |
| density | 4 g/mL at 25 °C(lit.) |
| Water Solubility | Insoluble |
| Merck | 14,6990 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7647-10-1(CAS DataBase Reference) |
| EPA Substance Registry System | Palladium chloride (PdCl2)(7647-10-1) |
| Safety Information |
| Hazard Codes | C,Xi,T+,T,Xn |
| Risk Statements | 34-43-40-28-41-37/38-25-22 |
| Safety Statements | 26-36/37/39-45-37/39-28-27-36/37-27/28 |
| RIDADR | UN 1789 8/PG 3 |
| WGK Germany | 2 |
| RTECS | RT3500000 |
| F | 3 |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28439090 |
| Hazardous Substances Data | 7647-10-1(Hazardous Substances Data) |

Details:
product information
|
Company information
Henan sunlake enterprise corporation is located in Henan Province , The central plain of China , Which enjoys favorable geogeaphical position and convenient transportion, The com[any was established in june. 1998 , until now having more than 18 years experience in manufacturing & exporting chemical raw material .
Sunlake is a professional manufacturer engaged in producing and selling chemicals,including Organic & inorganic chemicals , pigments & Dyestuffs , Water treatment chemicals , Food & FEED additives and others . these products have been being well exported to europe , southeast Asia , the Middle East , Africa , South America and some other countries and areas.
We sincerely welcome foreign friends to visit our plant for cooperation. With the idea of "quality first,credit priority, Excellent service", We are highly acknowledged by customers for good quality and competitive price. More importantly , the company has a strong R & D team, who are professional engineers and scholars with Ph. D. .So we are confident to serve you better with our high - quality products and professional team.
We are taking great efforts to provide our customers with demanded goods and professional services, and continuously improve our core ability of competition and get the momentum for sustainable development, and finally make us being a reliable and professional wupplier in international market.
We welcome any serious inquiries from all customers of the world, and sincerely hope to cooperate.

You Might Also Like
-
602-09-5 1,1'-Bi-2-naphthol (-)-2,2'-DIHYDROXY-1,1'-BINAPHTHYL
CAS NO:602-09-5
-
COA certificate products 95464-05-4 good quality best price
CAS NO:95464-05-4
-
Henan Sunlake Industrial 95464-05-4 good quality best price
CAS NO:95464-05-4
-
low price 95464-05-4 good quality best price
CAS NO:95464-05-4
-
HIGH PERFORMANCE 95464-05-4 good quality best price
CAS NO:95464-05-4
-
high purity 95464-05-4 good quality best price
CAS NO:95464-05-4
Related Searches
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View