| |
| Lecithin Basic information |
| |
| Lecithin Chemical Properties |
| Melting point |
>145°C (dec.) |
| density |
d424 1.0305 |
| Fp |
57 °C |
| storage temp. |
-20°C |
| solubility |
chloroform: 0.1 g/mL, slightly hazy, slightly yellow to deep orange |
| form |
solution |
| color |
Pale Brown to Yellow |
| Water Solubility |
NEGLIGIBLE |
| Merck |
14,5429 |
| BRN |
5209585 |
| Stability: |
Stable, but light, heat, moisture and air-sensitive. Incompatible with strong oxidizing agents. |
| InChIKey |
FWMYJLDHIVCJCT-VSZGHEPYSA-N |
| CAS DataBase Reference |
8002-43-5 |
| EPA Substance Registry System |
Lecithins (8002-43-5) |
| Hazard Codes |
|
| Risk Statements |
|
| Safety Statements |
|
| WGK Germany |
3 |
| RTECS |
OG7565000 |
| F |
1-8-10 |
| TSCA |
Yes |
| HS Code |
29239000 |
| Hazardous Substances Data |
8002-43-5(Hazardous Substances Data) |
| Toxicity |
LD50 oral in rat: > 8mL/kg |
| Provider |
Language |
| Lecithin |
English |
| ALFA |
English |
| |
| Lecithin Usage And Synthesis |
| Description |
Food-grade lecithin is obtained from soybeans and other plant sources. It is a complex mixture of acetone-insoluble phosphatides that consists chiefly of phosphatidyl choline, phosphatidyl etha nolamine, and phosphatidyl inositol, combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates. Refined grades of lecithin may contain any of these components in varying proportions and combinations depending on the type of fractionation used. In its oil-free form, the prepon-derance of triglycerides and fatty acids is removed and the product contains 90% or more of phosphatides representing all or certain fractions of the total phosphatide complex. The consistency of both natural grades and refined grades of lecithin may vary from plastic to fluid, depending upon free fatty acid and oil content, and upon the presence or absence of other diluents. Its color varies from light yellow to brown, depending on the source, on crop variations, and on whether it is bleached or unbleached. It is odorless or has a characteristic, slight nutlike odor and a bland taste. Edible diluents, such as cocoa butter and vegetable oils, often replace soybean oil to improve functional and flavor characteris tics. Lecithin is only partially soluble in water, but it readily hydrates to form emulsions. The oil-free phosphatides are soluble in fatty acids, but are practically insoluble in fixed oils. When all phosphatide fractions are present, lecithin is partially soluble in alcohol and practically insoluble in acetone. |
| Chemical Properties |
Lecithins vary greatly in their physical form, from viscous semiliquids to powders, depending upon the free fatty acid content. They may also vary in color from brown to light yellow, depending upon whether they are bleached or unbleached or on the degree of purity. When they are exposed to air, rapid oxidation occurs, also resulting in a dark yellow or brown color.
Lecithins have practically no odor. Those derived from vegetable sources have a bland or nutlike taste, similar to that of soybean oil. |
| Occurrence |
Lecithin is found in foods such as eggs, beef liver, and peanuts. Commercial sources are available |
| Uses |
Edible and digestible surfactant and emulsifier of natural origin. Used in margarine, chocolate and in the food industry in general. In pharmaceuticals and cosmetics. Many other industrial uses, e.g. treating leather and textiles. |
| Uses |
lecithin (hydrogenated) is an emulsifier. |
| Uses |
lecithin is a natural emollient, emulsifier, anti-oxidant, and spreading agent, lecithin is a hydrophilic ingredient that attracts water and acts as a moisturizer. generally obtained for cosmetic products from eggs and soybeans, it is found in all living organisms. |
| Uses |
egg lecithin is emollient and particularly recommended for sensitive skin. |
| Uses |
Lecithin is an emulsifier that is a mixture of phosphatides which are typically surface-active. it is now commercially obtained from soy- beans; previously it was obtained from egg yolk. it is used in marga- rine as an emulsifier and antispatter agent; in chocolate manufacture it controls flow properties by reducing viscosity and reducing the cocoa butter content from 3 to 5%; it is used as a wetting agent in cocoa powder, fillings, and beverage powders; an antisticking agent in griddling fat; and in baked goods to assist the shortening mix with other dough ingredients and to stabilize air cells. typical usage levels range from 0.1 to 1.0%. |
| Definition |
ChEBI: A glycerophosphocholine compound having O-acyl substituents at both the 1- and 2-positions of the glycerol. It is a major constituent of cell membranes. |
| Production Methods |
Lecithins are essential components of cell membranes and, in principle, may be obtained from a wide variety of living matter. In practice, however, lecithins are usually obtained from vegetable products such as soybean, peanut, cottonseed, sunflower, rapeseed, corn, or groundnut oils. Soybean lecithin is the most commercially important vegetable lecithin. Lecithin obtained from eggs is also commercially important and was the first lecithin to be discovered.
Vegetable lecithins are obtained as a by-product in the vegetable oil refining process. Polar lipids are extracted with hexane and, after removal of the solvent, a crude vegetable oil is obtained. Lecithin is then removed from the crude oil by water extraction. Following drying, the lecithin may be further purified.
With egg lecithin, a different manufacturing process must be used since the lecithin in egg yolks is more tightly bound to proteins than in vegetable sources. Egg lecithin is thus obtained by solvent extraction from liquid egg yolks using acetone or from freeze-dried egg yolks using ethanol (95%).
Synthetic lecithins may also be produced. |
| Pharmaceutical Applications |
Lecithins are used in a wide variety of pharmaceutical applications. They are also used in cosmetics and food products.
Lecithins are mainly used in pharmaceutical products as dispersing, emulsifying, and stabilizing agents, and are included in intramuscular and intravenous injections, parenteral nutrition formulations, and topical products such as creams and ointments.
Lecithins are also used in suppository bases, to reduce the brittleness of suppositories, and have been investigated for their absorption-enhancing properties in an intranasal insulin formulation. Lecithins are also commonly used as a component of enteral and parenteral nutrition formulations.
There is evidence that phosphatidylcholine (a major component of lecithin) is important as a nutritional supplement to fetal and infant development. Furthermore, choline is a required component of FDA-approved infant formulas. Other studies have indicated that lecithin can protect against alcohol cirrhosis of the liver, lower serum cholesterol levels, and improve mental and physical performance.
Liposomes in which lecithin is included as a component of the bilayer have been used to encapsulate drug substances; their potential as novel delivery systems has been investigated. This application generally requires purified lecithins combined in specific proportions.
Therapeutically, lecithin and derivatives have been used as a pulmonary surfactant in the treatment of neonatal respiratory distress syndrome. |
| Biochem/physiol Actions |
It also acts as a source of lipid messengers/ bioactive lipids including: lysophosphatidylcholine, diacylglycerol, phosphatidic acid, lysophosphatidylcholine, arachidonic acid and platelet activating factor. Phosphatidylcholine is produced in the liver by the CDP-choline (cytidine diphosphocholine) pathway. |
| Safety |
Lecithin is a component of cell membranes and is therefore consumed as a normal part of the diet. Although excessive consumption may be harmful, it is highly biocompatible and oral doses of up to 80 g daily have been used therapeutically in the treatment of tardive dyskinesia. When used in topical formulations, lecithin is generally regarded as a nonirritant and nonsensitizing material. The Cosmetic Ingredients Review Expert Panel (CIR) has reviewed lecithin and issued a tentative report revising the safe concentration of the material from 1.95% to 15.0% in rinse-off and leave-in products. They note, however, that there are insufficient data to rule on products that are likely to be inhaled. |
| storage |
Lecithins decompose at extreme pH. They are also hygroscopic and subject to microbial degradation. When heated, lecithins oxidize, darken, and decompose. Temperatures of 160–180°C will cause degradation within 24 hours.
Fluid or waxy lecithin grades should be stored at room temperature or above; temperatures below 10°C may cause separation.
All lecithin grades should be stored in well-closed containers protected from light and oxidation. Purified solid lecithins should be stored in tightly closed containers at subfreezing temperatures. |
| Purification Methods |
Lecithin from hen egg white is purified by solvent extraction and chromatography on alumina. It is suspended in H2O and kept frozen until required [Lee & Hunt J Am Chem Soc 106 7411 1984, Singleton et al. J Am Oil Chem Soc 42 53 1965]. For purification of commercial egg lecithin, see Pangborn [J Biol Chem 188 471 1951]. |
| Incompatibilities |
Incompatible with esterases owing to hydrolysis. |
| Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (inhalations; IM and IV injections; otic preparations; oral capsules, suspensions and tablets; rectal, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| |
| Lecithin Preparation Products And Raw materials |
|
| |
| |
| L-Tryptophan Chemical Properties |
| Melting point |
289-290 °C (dec.)(lit.) |
| alpha |
-31.1 º (c=1, H20) |
| Boiling point |
342.72°C (rough estimate) |
| density |
1.34 |
| refractive index |
-32 ° (C=1, H2O) |
| storage temp. |
2-8°C |
| solubility |
20% NH3: 0.1 g/mL at 20 °C, clear, colorless |
| pka |
2.46(at 25℃) |
| form |
powder |
| color |
White to yellow-white |
| PH |
5.5-7.0 (10g/l, H2O, 20℃) |
| optical activity |
[α]20/D 31.5±1°, c = 1% in H2O |
| Water Solubility |
11.4 g/L (25 ºC) |
| Merck |
14,9797 |
| BRN |
86197 |
| Stability: |
Stable. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey |
QIVBCDIJIAJPQS-VIFPVBQESA-N |
| CAS DataBase Reference |
73-22-3(CAS DataBase Reference) |
| NIST Chemistry Reference |
L-Tryptophan(73-22-3) |
| EPA Substance Registry System |
L-Tryptophan (73-22-3) |
| Provider |
Language |
| Indole-3-alanine |
English |
| ACROS |
English |
| SigmaAldrich |
English |
| ALFA |
English |
| |
| L-Tryptophan Usage And Synthesis |
| Description |
As an essential amino acid, L-Tryptophan is necessary for normal growth in infants and for nitrogen balance in adults, which cannot be synthesized from more basic substances in humans and other animals, suggesting that it is obtained only by intake of tryptophan or tryptophan-containing proteins for human body, which is particularly plentiful in chocolate, oats, milk, cottage cheese, red meat, eggs, fish, poultry, sesame, almonds, buckwheat, spirulina, and peanuts, etc. It can be used as a nutritional supplement for use as an antidepressant, anxiolytic, and sleep aid. Thus, L-Tryptophan can be used for depression, anxiety, sleep apnea, premenstrual syndrome and many other problems. Besides, it can also be used in managing pain tolerance and managing weight.
It works by increasing the levels of certain neurotransmitters in the brain called serotonin. People suffer from depression have an imbalance of serotonin and other brain chemicals. Thus, the increase of serotonin levels in the brain can improve symptoms of depression. L-Tryptopan serves as the precursor for the synthesis of serotonin, which is converted to serotonin in the body. As a result, the symptoms of depression and other problems are improved. |
| Overview |
It is also known as α-aminoindolylpropionic acid. Appearance: white to slightly yellowish white crystal or crystalline powder. No smell; Slight bitter taste. Melting temperature: 289 ° C (decomposition); soluble in water. Applications: as food fortifier, antioxidant; also used in medicine. It is manufactured from indole aldehyde, alternatively also be synthesized from trypsin decomposition and synthesis. 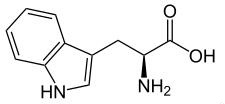 |
| Content analysis |
Accurately weigh about 300 mg sample and dissolve it in 3ml formic acid and 50ml glacial acetic acid; add 2 drops crystal violet test solution (TS-250), titrate with 0.1mol / L perchloric acid to the green end point or until the blue color completely disappears. Each Ml of 0.1 mol / L perchloric acid corresponds to 20.42 mg of L-tryptophan (C11H12N2O2). |
| Application |
Amino acids-type drug:
It can be used in amino acid infusion, being often combined with iron and vitamins. Its co-administration with VB6 can improve depression and prevention/treatment of skin disease; as a sleep sedative, it can be combined with L-dopa for the treatment of Parkinson's disease. It is carcinogenic to experimental animals; it may cause adverse reactions including nausea, anorexia and asthmas. Avoid combination with monoamine oxidase inhibitors.
Nutritional supplements:
Tryptophan contained in egg white protein, fish meat, corn meal and other amino acids are limited; content in cereals such as rice is also low. It can be combined with lysine, methionine and threonine for enhanced amino acids. It can be supplemented to corn product at the content of 0.02% tryptophan and 0.1% lysine, being capable of significantly improving the protein potency. |
| References |
#
//www.medicinenet.com/tryptophan-oral_capsule_tablet/article.htm
//bodyandhealth.canada.com/drug/getdrug/apo-tryptophan
# |
| Chemical Properties |
White to off-white crystalline powder |
| Chemical Properties |
Appearance: white crystalline powder. Odorless, slightly bitter taste;
Solubility: slightly soluble in water (1.14%, 25 ℃), insoluble in ethanol; soluble in dilute acid or dilute alkali. mp 289 ° C (decomposition).
Isoelectric point: 5.89. [α] D + 2.8 (5 mol / L HCl), [α] D + 6.2 (0.5 mol / L NaOH).
It can be colored upon long-term exposure to light. Its co-heating reaction with water will generate a small amount of indole while heating in the presence of sodium hydroxide and copper sulfate will produce a large amount of indole. It is stable upon heating together with acid in the dark, but is easily to be decomposed when heated together with other amino acids, carbohydrates and aldehydes. L-tryptophan will be completely decomposed when applying acid to break down the protein. |
| Uses |
adrenergic agonist |
| Uses |
L-tryptophan is an essential amino acid which is necessary for normal growth in infants and for nitrogen balance in adults. It acts as a natural dietary supplement and used as an antidepressant, anxiolytic and sleep aid. It is used as a precursor to niacin, indole alkaloids and serotonin. It acts as an important intrinsic fluorescent probe, which finds to estimate the nature of microenvironment of the tryptophan. |
| Uses |
tryptophan is one of the 21 amino acids comprising a protein. Tryptophan is a component of the skin’s natural moisturizing factors. |
| Definition |
ChEBI: The L-enantiomer of tryptophan. |
| Brand name |
Ardeytropin;Kalma;Optimax;Sedanoct. |
| World Health Organization (WHO) |
L-tryptophan, an essential aminoacid and precursor of serotonin, was introduced into medicine in 1963 for the treatment of depression and sleep disorders. Its effectiveness in these conditions has, however, never been convincingly demonstrated. It is also widely used in dietary supplements, parenteral nutrition preparations and dietary products for children with phenylketonuria. In 1989, reports from the USA showed an association between the consumption of L-tryptophan containing preparations and the development of eosiniphilia-myalgia syndrome (EMS), a condition characterized by intense eosinophilia, severe muscle and joint pain, swelling of the arms and legs, skin rashes and possible fever. Some of the reported cases have been fatal. Since it is not yet clear whether L-tryptophan itself or an unidentified contaminant is the cause of the EMS, many drug regulatory authorities have suspended the marketing authorization of products containing tryptophan pending further investigation, whereas others have withdrawn these products or restricted their use. |
| Synthesis Reference(s) |
The Journal of Organic Chemistry, 49, p. 3711, 1984 DOI: 10.1021/jo00194a008 |
| General Description |
White powder with a flat taste. An essential amino acid; occurs in isomeric forms. |
| Air & Water Reactions |
Slightly soluble in water. |
| Reactivity Profile |
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
| Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition L-Tryptophan emits toxic fumes. |
| Fire Hazard |
Flash point data for L-Tryptophan are not available. L-Tryptophan is probably combustible. |
| Biochem/physiol Actions |
Tryptophan (Trp) is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Increased Trp availability is necessary for the regulation of mood, cognition and behaviour. It is hypothesised that L-Trp might be useful in inducing sleep in healthy adults against the normal circadian rhythm. Trp uptake by the brain depends on the plasma ratio of Trp to all of the other LNAAs (large neutral amino acids). Higher the Trp:LNAAs ratio, greater is the Trp uptake. |
| Safety Profile |
Moderately toxic by intraperitoneal route. Experimental teratogenic and reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of NOx. |
| Purification Methods |
Crystallise L-tryptophan from H2O/EtOH, wash it with anhydrous diethyl ether and dry it at room temperature in a vacuum over P2O5. It sublimes at 220-230o/0.03mm with 99% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Cox & King Org Synth Coll Vol II 612 1943, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2316-2345 1961, Beilstein 22 IV 6765.] |
| |
| L-Tryptophan Preparation Products And Raw materials |
|
| |
| L-Norvaline Chemical Properties |
| Melting point |
>300 °C(lit.) |
| alpha |
24.5 º (c=10, 6 N HCl) |
| Boiling point |
222.9±23.0 °C(Predicted) |
| density |
1.2000 (estimate) |
| refractive index |
25 ° (C=10, 6mol/L HCl) |
| storage temp. |
Store below +30°C. |
| solubility |
48.7g/l |
| pka |
2.32(at 25℃) |
| form |
Fine Crystalline Powder |
| color |
White |
| Water Solubility |
10.5 g/100 mL (18 ºC) |
| Merck |
14,6716 |
| BRN |
1721162 |
| CAS DataBase Reference |
6600-40-4(CAS DataBase Reference) |
| NIST Chemistry Reference |
L-Norvaline(6600-40-4) |
| Provider |
Language |
| L(+)-2-Aminovaleric acid |
English |
| ACROS |
English |
| SigmaAldrich |
English |
| ALFA |
English |
| |
| L-Norvaline Usage And Synthesis |
| Chemical Properties |
white to light yellow crystal powder |
| Uses |
L-Norvaline is a protein amino acid used as a skin-conditioning and anti-static agent. |
| Uses |
L-Norvalineis used in method for reducing misincorporation of non-canonical branched-chain Amino Acids into recombinant proteins. |
| Definition |
ChEBI: A 2-aminopentanoic acid that has S-configuration. |
| Biochem/physiol Actions |
L-Norvaline enhances NO production from activated macrophages. |
| Purification Methods |
Crystallise norvaline from aqueous EtOH or water. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2390-2399 1961, Beilstein 4 III 1331-1333, 4 IV 128, 2629.] |
| |
| L-Norvaline Preparation Products And Raw materials |
|
| |
| |
| L-Ornithine Chemical Properties |
| Melting point |
140°C |
| alpha |
D25 +11.5° (c = 6.5) |
| Boiling point |
244.08°C (rough estimate) |
| density |
1.1740 (rough estimate) |
| refractive index |
1.4496 (estimate) |
| storage temp. |
under inert gas (nitrogen or Argon) at 2–8 °C |
| pka |
1.705(at 25℃) |
| CAS DataBase Reference |
70-26-8(CAS DataBase Reference) |
| EPA Substance Registry System |
L-Ornithine (70-26-8) |
| Toxicity |
sce-hmn-lym 10 mg/L MUREAV 372,75,1996 |
| |
| L-Ornithine Usage And Synthesis |
| Chemical Properties |
Crystals from alcoholether. Soluble in water and alcohol. |
| Uses |
Biochemical research; medicine. |
| Uses |
hepatoprotectant, anticholesteremic |
| Definition |
ChEBI: An optically active form of ornithine having L-configuration. |
| Synthesis Reference(s) |
Canadian Journal of Chemistry, 31, p. 1060, 1953 DOI: 10.1139/v53-139 |
| Safety Profile |
Mutation data reported. When heated to decomposition it emits toxic vapors of NOx. |
| Purification Methods |
Crystallise L-ornithine from water containing 1mM EDTA (to remove metal ions). [Perrin J Chem Soc 3125 1958, Rivard Biochemical Preparations 3 97 1955, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2477-2491 1961, Beilstein 4 III 1346, 4 IV 2644.] |
| |
| L-Ornithine Preparation Products And Raw materials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
