| Product Name: |
Potassium sorbate |
| Synonyms: |
SORBIC ACID K SALT;potassiumsalt,(e,e)-4-hexadienoicacid;potassiumsalt,(e,e)-sorbicaci;potassiumsorbate(e);(E,E)-hexadienoic acid, potassium salt;2,4-potassium hexadienoic acid;potassium (E,E)-hexa-2,4-dienoate;POTASSIUM SORBATE, GRAN FCC/ USP/NF |
| CAS: |
24634-61-5 |
| MF: |
C6H7KO2 |
| MW: |
150.22 |
| EINECS: |
246-376-1 |
| Product Categories: |
Food additive;FOOD ADDITIVES;Food & Feed ADDITIVES;Classes of Metal Compounds;K (Potassium) Compounds (excluding simple potassium salts);Typical Metal Compounds |
| Mol File: |
24634-61-5.mol |
 |
| |
| Potassium sorbate Chemical Properties |
| Melting point |
270 °C |
| density |
1,361 g/cm3 |
| vapor pressure |
<1 Pa (20 °C) |
| FEMA |
2921 | POTASSIUM SORBATE |
| storage temp. |
2-8°C |
| solubility |
H2O: 1 M at 20 °C, clear, colorless to faintly yellow |
| form |
Powder |
| color |
White to light cream |
| Odor |
Odorless |
| PH |
7.8 (H2O, 20.1℃) |
| PH Range |
8 - 11 at 580 g/l at 20 °C |
| Water Solubility |
58.2 g/100 mL (20 ºC) |
| Merck |
14,7671 |
| BRN |
5357554 |
| Stability: |
Stable. Incompatible with strong oxidizing agents. |
| InChIKey |
CHHHXKFHOYLYRE-STWYSWDKSA-M |
| CAS DataBase Reference |
24634-61-5(CAS DataBase Reference) |
| EPA Substance Registry System |
Potassium sorbate (24634-61-5) |
| Hazard Codes |
Xi,C,T,F |
| Risk Statements |
36/37/38-35-22 |
| Safety Statements |
26-36-45-36/37/39 |
| WGK Germany |
1 |
| RTECS |
WG2170000 |
| Autoignition Temperature |
>150 °C |
| TSCA |
Yes |
| HS Code |
2916 19 95 |
| Toxicity |
LD50 orally in Rabbit: 3800 mg/kg |
| Provider |
Language |
| SigmaAldrich |
English |
| ACROS |
English |
| ALFA |
English |
| |
| Potassium sorbate Usage And Synthesis |
| Overview |
Potassium sorbate is petitioned for use in organic livestock production as mold inhibitor[1-3]. Sorbic acid was first discovered in the Mountain Ash Tree (Sorbus aucuparia or Sorbus americana). Today most potassium sorbate is made synthetically. Potassium sorbate is a naturally occurring unsaturated fatty acid and is completely safe with regard to health and has the lowest allergenic potential of all food preservatives. Potassium sorbate was also petitioned for use in liquid livestock medications primarily aloe vera juice as a substitute for antibiotics and other various hormones[1-4].
The use of chemical food preservatives, except for salts, sugars, spices, vinegar, etc., was not very widespread until the last 200 years. Progress in the development of food preservatives has not been steady. With a view to developing more effective, simpler, and less expensive means of food preservation, many chemicals having strong antimicrobial properties were initially utilized for food preservation but were subsequently abandoned when their undesirable physiological and biochemical properties were discovered. For example, boric acid, salicyclic acid, creosote, and formaldehyde, which were utilized as preservatives in foods during the 19th century, are no longer used. On the other hand, sorbic acid (SA), benzoic acid, p-hydroxy benzoic acid esters, and sulfur dioxide have proved very useful in various food preservation applications and their use has been officially permitted in almost all countries of the world [1,4].
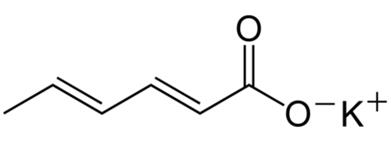
Figure 1 the chemical structure of potassium sorbate |
| Chemical Properties |
Chemically, sorbic acid is a straight chain, alpha beta-unsaturated, trans-trans 2,4 hexadienoic monocarboxylic acid (CH3-CH = CH-CH = CH-COOH). It has a molecular weight of 112[5] and a pKa value of 4.75. At room temperature sorbic acid is a white crystalline solid with a melting point range of 132°-137°C.
Its solubility in water at 25°C is 0.16% while that of its potassium salt is over 50%.
This higher solubility renders potassium sorbate a preferred form of sorbic acid in foods. In oils, however, sorbic acid is more soluble than the potassium salt.
Sorbic acid was first isolated from oil of unripened rown berries (sorbapple or mountain ash berry) by A. W. Hoffmann in 1859. The compound was named after the scientific name of mountain ash {Sorbus aucuparia}, which is the parent plant of rown berry[6, 7]. The chemical structure of sorbic acid was elucidated during 1870-1890 and it was synthesized in 1900 by Doebner by condensation of crotonalhyde and malonic acid[6]. |
| Application |
When dissolved in water, potassium sorbate ionizes to form sorbic acid which is effective against yeasts, molds, and select bacteria, and is widely used at 250 ppm to 1000 ppm levels in cheeses, dips, yogurt, sour cream, bread, cakes, pies and fillings, baking mixes, doughs, icings, fudges, toppings, beverages, margarine, salads, fermented and acidified vegetables, olives, fruit products, dressings, smoked and salted fish, confections and mayonnaise. Therefore, it is generally used as a powerful food preservative. |
| Antimicrobial effect |
Antimicrobial properties of sorbic acid were discovered independently in 1939 and 1940 by Muller and Gooding in Germany and the USA, respectively[2, 10]. After this discovery, sorbic acid and its salts were tested and used in a variety of consumer products for inhibition of yeast and molds and certain bacteria. But its use as a food preservative had to wait until 1950 when commercial production commenced. Initially sorbates were known to be effective inhibitors of yeast and molds, and less so of bacteria. In 1974 Tompkin et al.[8] reported that addition of 0.1 % potassium sorbate to uncured sausages delayed the growth of Salmonella spp. and Staphylococcus aureus as well as growth and toxin production by Clostridium botulinum. Following these findings, extensive studies were undertaken on the potential use of sorbic acid or its salts as antibotulinal agents and preservatives in various types of meats and meat products. These compounds were tested in combination with low levels of sodium nitrite for the preservation of cured meats and the reduction of potentially carcinogenic nitrosamine in products such as bacon[9, 10]. Most recently sorbic acid has played a very important role in the development of intermediate-moisture foods. The water activity of these foods is low enough to control the growth of bacteria but not growth of yeast and molds; therefore, sorbic acid is used as a very effective antimycotic agent in these products[11]. Sorbic acid and its salts are also being used as one of the various "hurdles" employed to control microbial growth in intermediate moisture foods[12].
Unfortunately, grain and feed provides an ideal environment for molds to proliferate. Raw materials or feeds in bulk storage are rich sources of energy, proteins and moisture and, thus, are highly conducive to mold growth[13]. Potassium sorbate is the potassium salt of sorbic acid, and is much more soluble in water than the acid. Potassium sorbate will produce sorbic acid once it is dissolved in water and is the most widely used food preservative in the world. It is effective up to pH 6.5 but effectiveness increases as the pH decreases. Potassium sorbate has about 74% of the antimicrobial activity of the sorbic acid, thus requiring higher concentrations to obtain the same results that pure sorbic acid provides. Potassium sorbate is effective against yeasts, molds, and select bacteria, and is widely used at 0.025 to 0.10 % levels in cheeses, dips, yogurt, sour cream, bread, cakes, pies and fillings, baking mixes, doughs, icings, fudges, toppings, beverages, margarine, salads, fermented and acidified vegetables, olives, fruit products, dressings, smoked and salted fish, confections and mayonnaise. Maximum level allowable by law is 0.1%. It is important to know that the addition of sodium benzoate and/or potassium sorbate to a food product will raise the pH by approximately 0.1 to 0.5 pH units depending on the amount, pH, and type of product. Additional adjustment of the pH might be needed to keep the pH at a safe level[14]. |
| Immunomodulatory effect |
While one recent study reported that potassium sorbate can contribute to the activation of inflammatory pathways[15], other studies indicate that sorbate is primarily anti-inflammatory in vivo and acts to downregulate many immune signaling pathways responsible for inflammation, glial cell activation, switching of T-helper cells, modulation of regulatory T cells, cell-to-cell contact, and migration[16]. These latter, anti-inflammatory effects of sorbate would appear to resemble those of sodium salicylate, the active metabolite of the well-known nonsteroidal anti-inflammatory drug aspirin (acetylsalicylic acid). In mouse microglia, sorbate inhibits NF-kappaB activation, modulates the mevalonate pathway, and suppresses the activation of p21ras[17]. Recently, sorbate administration was shown to induce the expression of TGF-beta in splenocytes and also to upregulate regulatory T cells during experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis (MS)[18]. Interleukin 4 (IL-4) is known to improve the clinical manifestations in this animal model of MS, and the administration of sorbate to human subjects has been shown to induce IL-4 production in their peripheral blood mononuclear cells. Sorbate might therefore deserve consideration as a useful candidate for conjunctive therapy in treating MS, the most common human demyelinating disease of the central nervous system. |
| Health concern |
Until recently, the extensive use of sorbate salts for large-scale food and drink preservation was regarded as completely safe. Claims to this effect are often still issued by the organizations that represent the soft drink industry. Such statements reflect the long history of apparently safe use of these preservatives and a safety testing that has largely focused on the maximum levels of these compounds that could be tolerated without adverse effects in the diet of laboratory animals[19]. The maximum levels of benzoate and sorbate permitted in food and drinks are based on these studies. Ever since these original safety tests, there have been dramatic advances in the technologies available to investigate damage to cells and tissues, providing opportunities for much deeper investigation of the effects of these additives and the consequences of their long-term, large-scale dietary consumption. Indeed, we are now aware of mechanisms of damage to biological systems that were completely unknown at the time of much of the original safety testing of these preservatives. There are more and more concerns regarding the safety use of sorbate.
Sorbic acid and potassium sorbates have a very low mammalian toxicity. There is a general consensus that they are intrinsically devoid of carcinogenic activity, but have the potential to undergo a conversion to potential mutagens. In tests on Syrian hamster embryo fibroblasts, Chinese hamster ovary cells, or bone marrow cells, no genotoxic or cell-transforming activity was detected with freshly prepared sodium sorbate solution. However, products of sodium sorbate with genotoxic and cell-transforming properties were formed under conditions of heating and storage. Sorbate can undergo oxidation to 4,5-oxohexanoate[20] and oxidized potassium sorbate can react with ascorbic acid in the presence of ferrous iron[21]. Much attention has been focused on the reactions between sorbate and nitrite at pH2-4.2, conditions that mimic the gastric environment. Among the products of such reactions are the mutagenic agents 1,4dinitro-2-methylpyrrole and ethylnitrolic acid[22].
There is one report of hepatoma arising from the feeding of mice on a diet of very high (15% w/v) sorbic acid, this being correlated with a depletion of the levels of reduced glutathione (GSH) in the mouse liver. Causation of this hepatoma was attributed to the oxidative stress caused by the depleted GSH pool, together with the gradual production of various mutagens in the intestine, mutagens that following their absorption were transferred to the liver where they were, in turn, metabolically activated to carcinogenic compounds[23].
There are also concerns regarding the effect of sorbate on the mitochondrial function. The study of artificial phospholipid bilayer membranes has revealed that sorbate cause significant increases in membrane conductance and proton permeability, mainly by acting as lipid soluble anions at neutral pH and as proton carriers when the pH approximates to the pK of the acid[24]. The action of sorbate on membranes is thought to be exerted mainly through a disruptive effect on membrane structure[25]. This weak acid has a pronounced effect on mitochondrial function, generating a decreased electron flow from substrate dehydrogenases to ubiquinone that in turn increases free electron “leakage” from the respiratory chain, electrons that then combine with molecular oxygen to produce superoxide (O2•−). A number of other moderately lipophilic compounds of food and drink relevance, notably ethanol and certain plant essential oils, including the widely used menthol generate ROS in a similar manner. |
| References |
- D. P. Smith and N. J. Rollin, Food Res., 19, 59 (1954).
- J. W. Boyd and H. L. A. Tarr, Food Technol., 9, 411 (1955).
- C. M. Gooding, D. Melnick, R. L. Lawrence, and F. H. Luckman, Food Res., 20, 639 (1955).
- J. N. Sofos and F. F. Busta, J. Food Prot., 44, 614 (1981).
- E. Lueck, Food Additiv. Contaminants, 7, 711 (1990).
- E. Lueck, Int. Flavours Food Additiv., 7, 122 (1976).
- E. Lueck, "Antimicrobial Food Additives," Springer-Verlag, New York, 1980
- R. B. Tompkin, L. N. Christiansen, A. B. Shaparis, and H. Bolin, Appl. Microbiol., 28, 262 (1974).
- National Academy of Sciences, National Academy Press, Washingtin, DC, 1981.
- M. C. Robach and J. N. Sofos, J. Food Prot., 45, 374 (1982).
- L. E. Erickson, J. Food Prot., 45, 484 (1982).
- S. L. Boylan, K. A. Acott, and T. P. Labuza, J. Food Sci., 41, 918 (1976).
- //www.kemin.com/livestock-feed-ingredients/mold-antimicrobials.shtml
- //www.nysaes.cornell.edu/fst/fvc/Venture/venture2_chemical.html
- Raposa B, et al 2016. Physiol Intl 103(3):334–43.
- Pahan K. 2011.Immunopharmacol Immunotoxicol 33(4):586–93.
- Brahmachari S, Jana A, Pahan K. 2009. J Immunol 183(9):5917–27.
- Kundu M, et al J Immunol 197(8): 3099–110.
- Final report on the safety assessment of sorbic acid and potassium sorbate. 1988. J Am College Toxicol 7(6):837–80.
- Jung R, et al 1992. Food Chem Toxicol 30:1–7.
- Kitano K et al 2002. Food Chem Toxicol 40(11):1589–94.
- Perez-Prior MT, et al 2008. J Agric Food Chem 56(24):11824–9.
- Nishimaki-Mogami T, Tanaka A, Minegishi K, Takahashi A. 1991. Biochem Pharmacol 42(2):239–46.
- Gutknecht J. 1992. Mol Cell Biochem 114(1–2):3–8.
- Stratford M, et al 2013. Intl J Food Microbiol 161(3):164–71.
|
| Description |
Potassium sorbate is a white crystalline powder. It is a potassium salt of sorbic acid.It was originally discovered in the 1850’s, and was derived from the Mountain Ash Tree. Today, potassium sorbate is synthetically created. Potassium sorbate is a good food preservatives, fully degradable, similar to fatty acids found naturally in foods. It is used to slow the growth of molds and yeasts in foods. It is commonly found in margarine, wines, cheeses, yogurts, soft drinks, and baked goods. Potassium sorbate has been used has a food preservative for many years. There have been extensive long-term tests that have confirmed its safety and it is on the Center for Science in the Public Interest list of safe additives. |
| Chemical Properties |
Potassium sorbate occurs as a white crystalline powder with a faint, characteristic odor. |
| Chemical Properties |
Potassium sorbate is the potassium salt of sorbic acid, chemical formula C6H7KO2. Its primary use is as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal care products. Commercial sources are now produced by the condensation of crotonaldehyde and ketene (Ashford, 1994). |
| Chemical Properties |
Potassium sorbate is produced by neutralizing potassium hydroxide with sorbic acid, an unsaturated carboxylic acid that occurs naturally in some berries. The colourless salt is very soluble in water (58.2% at 20°C). |
| Physical properties |
Colorless or white crystalline solid; density 1.36 g/cm3; decomposes at 270°C; soluble in water, 58 g/100 g solution; moderately soluble in alcohol. |
| Uses |
Potassium sorbate is used to inhibit molds and yeasts in many foods, such as cheese, wine, yogurt, dried meats, apple cider, soft drinks and fruit drinks, and baked goods. It can also be found in the ingredients list of many dried fruit products. In addition, herbal dietary supplement products generally contain potassium sorbate, which acts to prevent mold and microbes and to increase shelf life, and is used in quantities at which there are no known adverse health effects, over short periods of time.Labeling of this preservative on ingredient statements reads as "potassium sorbate" and or "E202". Also, it is used in many personal care products to inhibit the development of microorganisms for shelf stability. Some manufacturers are using this preservative as a replacement for parabens.
Also known as "wine stabilizer", potassium sorbate produces sorbic acid when added to wine. It serves two purposes. When active fermentation has ceased and the wine is racked for the final time after clearing, potassium sorbate will render any surviving yeast incapable of multiplying. Yeast living at that moment can continue fermenting any residual sugar into CO2 and alcohol, but when they die no new yeast will be present to cause future fermentation. |
| Uses |
Sorbic acid is a naturally occurring polyunsaturated fat that has antimicrobial properties. That means that it helps to prevent the growth of molds, yeasts, and fungus. Potassium sorbate is found in many food products, especially those which are meant to be stored and eaten at room temperature. This helps to ward off particles such as mold or fungus that can cause foods to spoil or make people sick. Baked goods, processed fruits and vegetables or dairy products frequently contain this product. When brewing wine, yeast is used to convert sugar to alcohol. This process is called fermentation. When the wine reaches the desired flavour and body, you want to stop the yeast from growing. Potassium sorbate is added to inhibit yeast growth. |
| Uses |
As mold and yeast inhibitor, like sorbic acid, especially where greater soly in water is desirable. |
| Uses |
Sorbic acid and its potassium salt is commonly employed as food preservative in wide range of foodstuffs, such as cheese, pickles, sauces and wines.
Potassium sorbate is a food grade preservative generally regarded as safe (GRAS) worldwide. It is the inactive salt of sorbic acid. It readily dissolves in water where it converts to sorbic acid, its active form, at a low pH. Sorbic acid is very pH dependent. While it shows some activity up to pH 6 (about 6%), it is most active at pH 4.4 (70%). At pH 5.0 it is 37% active. As sorbic acid, it is considered to be active against mold, fair against yeast and poor against most bacteria. Sorbic acid is an unsaturated fatty acid and as such is subject to oxidation (use of an antioxidant like Mixed Tocopherols T50 is recommended). It is also sensitive to UV light and may turn yellow in solution. Gluconolactone is reported to stabilize potassium sorbate against discoloration and darkening in aqueous solutions and may be useful in stabilizing sorbic acid in the water phase of a product. |
| Definition |
ChEBI: A potassium salt having sorbate as the counterion. |
| Production Methods |
Potassium sorbate is prepared from sorbic acid and potassium hydroxide. |
| Production Methods |
Potassium sorbate is produced by reacting sorbic acid with an equimolar portion of potassium hydroxide. The resulting potassium sorbate may be crystallized from aqueous ethanol.
Most of the sorbic acid is generally prepared by a process comprising the steps of reacting crotonaldehyde with ketene in the presence of a catalyst (e.g., a fatty acid salt of zinc) to yield a polyester, and hydrolyzing the polyester with an acid or an alkali, or decomposing the polyester in a hot water. |
| Preparation |
Potassium sorbate is prepared by reacting potassium hydroxide with sorbic acid, followed by evaporation and crystallization:CH3CH=CHCH=CHCOOH + KOH →CH3CH=CHCH=CHCOOK + H2O. |
| General Description |
Sorbates have been reported to be less toxic than benzoate and have been classified as “Generally Recognized as Safe” (GRAS) additives by the U.S. Food and Drug Administration (FDA).Sorbic acid is metabolised to mainly to carbon dioxide. While the minor amounts are converted to trans,trans-muconic acid (ttMA), which is excreted unchanged into the urine. Urinary ttMA is a biomarker for the occupational and environmental exposure to benzene. Ability of trans,trans-2,4-Hexadienoic acid potassium salt (Sorbic acid potassium salt, Potassium sorbate) to induce chromosome aberrations, sister chromatid exchanges (SCE) and gene mutations in cultured Chinese hamster V79 cells has been examined.Potassium sorbate is reported to be less genotoxic than the sodium salt analog.
|
| Pharmaceutical Applications |
Potassium sorbate is an antimicrobial preservative, with antibacterial and antifungal properties used in pharmaceuticals, foods, enteral preparations, and cosmetics. Generally, it is used at concentrations of 0.1–0.2% in oral and topical formulations, especially those containing nonionic surfactants. Potassium sorbate has been used to enhance the ocular bioavailability of timolol.
Potassium sorbate is used in approximately twice as many pharmaceutical formulations as is sorbic acid owing to its greater solubility and stability in water. Like sorbic acid, potassium sorbate has minimal antibacterial properties in formulations above pH 6. |
| Toxicology |
Potassium sorbate is a skin, eye and respiratory irritant. Although some research implies it has a long term safety record, in vitro studies have shown that it is both genotoxic and mutagenic to human blood cells. Potassium sorbate is found to be toxic to human DNA in peripheral blood lymphocytes (type of white blood cells), and hence found that it negatively affects immunity. It is often used with ascorbic acid and iron salts as they increase its effectiveness but this tends to form mutagenic compounds that damage DNA molecules.
Potassium sorbate exhibits low toxicity with LD50 (rat, oral) of 4.92 g / kg, similar to that of table salt . Typical usage rates of potassium sorbate are 0.025 % to 0.1 % (see sorbic acid), which in a 100 g serving yields intake of 25 mg to 100 mg. Acceptable daily intakes for human is 12.5 mg / kg, or 875 mg daily for an average adult (70 kg), according to FAO/World Health Organization Expert Committee on Food Additives. |
| Safety |
Potassium sorbate is used as an antimicrobial preservative in oral and topical pharmaceutical formulations and is generally regarded as a relatively nontoxic material. However, some adverse reactions to potassium sorbate have been reported, including irritant skin reactions which may be of the allergic, hypersensitive type. There have been no reports of adverse systemic reactions following oral consumption of potassium sorbate.
The WHO has set an estimated total acceptable daily intake for sorbic acid, calcium sorbate, potassium sorbate, and sodium sorbate expressed as sorbic acid at up to 25 mg/kg body-weight.
(mouse, IP): 1.3 g/kg
(rat, oral): 4.92 g/kg
See also Sorbic Acid. |
| storage |
Potassium sorbate is more stable in aqueous solution than sorbic acid; aqueous solutions may be sterilized by autoclaving.
The bulk material should be stored in a well-closed container, protected from light, at a temperature not exceeding 40°C. |
| Incompatibilities |
Some loss of antimicrobial activity occurs in the presence of nonionic surfactants and some plastics. |
| Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (nasal sprays; oral capsules, solutions, suspensions, syrups, tablets; topical creams and lotions). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| |
| Potassium sorbate Preparation Products And Raw materials |
|
|
|
|
|
|
|
|
|
|
|
|
|
