| |
| Potassium chloride Chemical Properties |
| Melting point |
770 °C (lit.) |
| Boiling point |
1420°C |
| density |
1.98 g/mL at 25 °C (lit.) |
| refractive index |
n20/D 1.334 |
| Fp |
1500°C |
| storage temp. |
2-8°C |
| solubility |
H2O: soluble |
| form |
random crystals |
| color |
White |
| Specific Gravity |
1.984 |
| Odor |
Odorless |
| PH Range |
7 |
| PH |
5.5-8.0 (20℃, 50mg/mL in H2O) |
| Water Solubility |
340 g/L (20 ºC) |
| Sensitive |
Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.02
λ: 280 nm Amax: 0.01 |
| Merck |
14,7621 |
| Sublimation |
1500 ºC |
| BRN |
1711999 |
| Stability: |
Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Hygroscopic. |
| InChIKey |
WCUXLLCKKVVCTQ-UHFFFAOYSA-M |
| CAS DataBase Reference |
7447-40-7(CAS DataBase Reference) |
| NIST Chemistry Reference |
Potassium chloride(7447-40-7) |
| EPA Substance Registry System |
Potassium chloride (7447-40-7) |
| Provider |
Language |
| Potassium chloride |
English |
| ACROS |
English |
| |
| Potassium chloride Usage And Synthesis |
| Chemical Properties |
Potassium chloride, KCI, also known as potassium muriate and sylvite, is a colorless crystalline solid with a salty taste that melts at 776°C (1420 OF). It is soluble in water, but insoluble in alcohol. Potassium chloride is used in fertilizers, pharmaceuticals, photography, and as a salt substitute.

potassium chloride powder |
| Uses |
Potassium chloride (KCl) is used in drug preparations and as a food additive and chemical reagent. It is possible to reduce the sodium in your diet by substituting potassium chloride for table salt (sodium chloride), which may be healthier. Molten potassium chloride is also used in the electrolytic production of metallic potassium. KCl is also found in seawater brine and can be extracted from the mineral carnallite. |
| Description |
Potassium chloride (KCl) is a metal halide salt that is used in a variety of areas. The dominant application of potassium chloride is to serve as a fertilizer, which offers potassium to plants and prevents them from certain diseases. Besides, it can be applied in food and medical industry. As a treatment for hypokalemia, potassium chloride pills are taken to balance the blood's potassium levels and prevent potassium deficiency in the blood. In food industry, it serves as a electrolyte replenisher and a good salt substitute for food, as well as a firming agent to give consistent texture to food, thus to strengthen its structure. |
| Chemical Properties |
Potassium chloride occurs as odorless, colorless crystals or a white crystalline powder, with an unpleasant, saline taste. The crystal lattice is a face-centered cubic structure. |
| Chemical Properties |
Potassium chloride is colorless or white crystals; strong saline taste. Occurs naturally as sylvite. Soluble in water; slightly soluble in alcohol. Sp. gr. 1.987; sublimes at 1500°C; noncombustible; low toxicity. Used in fertilizers, as a source of potassium salts; pharmaceutical preparations; photography; spectroscopy; plant nutrient; salt substitute; laboratory reagent. |
| Uses |
Potassium Chloride is a nutrient, dietary supplement, and gelling agent that exists as crystals or powder. it has a solubility of 1 g in 2.8 ml of water at 25°c and 1 g in 1.8 ml of boiling water. hydrochloric acid, and sodium chloride and magnesium chloride diminish its solubility in water. it is used as a salt substitute and mineral supple- ment. it has optional use in artificially sweetened jelly and preserves. it is used as a potassium source for certain types of carrageenan gels. it is used to replace sodium chloride in low-sodium foods. |
| Uses |
potassium chloride is a laboratory reagent used to increase product viscosity in cosmetic and pharmaceutical preparations. |
| Uses |
Potassium chloride (KCl), commonly referred to as muriate of potash, is the most common source of potash (K2O), and accounts for about 95 % of world potash production. Virtually all (90 %) commercial potash is extracted from natural sources of potassium salt deposits occurring in thin beds in large salt basins formed by the evaporation of ancient seas. Present-day salt lakes and natural brines represent about 10 % of total recoverable potash. Extraction is followed by milling, washing, screening, flotation, crystallization, refining and drying.
More than 90 % of the total KCl consumption is used for fertilizer production. Production of potassium hydroxide accounts for more than 90 % of the non-fertilizer or industrial use of KCl. KOH is also used in the production of some agricultural-grade liquid fertilizers. uses of KCl include:
- Potassium chloride (KCl) is inorganic salt used for making fertilizers, since the growth of many plants is limited by their potassium intake. Potassium in plants is important for the osmotic and ionic regulation, plays a key role in the water homeostasis and is closely connected with processes involved in the protein synthesis.
- In photography. In buffer solutions, electrode cells.
- Potassium chloride may be used for the preparation of phosphate buffered saline, and for the extraction and solubilization of proteins.
- Used in buffer solutions, medicine, scientific applications, and food processing.
- Used in nutritent; gelling agent; salt substitute; yeast food.
- food/foodstuff additives: KCl is used as a nutrient and/or dietary supplement food additive. KCl also serves as a potassium supplement of animal feed.
- pharmaceutical products: KCl is an important therapeutic agent, which is used mainly in the treatment of hypokalemia and associated conditions. Hypokalemia (potassium deficiency) is a potentially fatal condition in which the body fails to retain sufficient potassium to maintain health.
- laboratory chemicals: KCl is used in electrode cells, buffer solutions, and spectroscopy.
- drilling mud for oil production industry: KCl is used as conditioner in oil drilling muds and as a shale stabilizer to prevent swelling.
- flame retardants and fire preventing agents: KCl is used as a component in dry chemical fire extinguisher.
- anti-freezing agents: KCl is used to melt ice on streets and driveways.
|
| Uses |
About 4-5% of potash production is used in industrial applications (UNIDOIFDC, 1998). In 1996, the world supply of industrial grade potash was close to 1.35 Mt K2O. This industrial material is 98-99% pure, compared with the agricultural potash specification of 60% K2O minimum (equivalent to 95% KCl). Industrial potash should contain at least 62% K2O and have very low levels of Na, Mg, Ca, SO4 and Br. This high-grade potash is produced by only a few producers in worldwide.
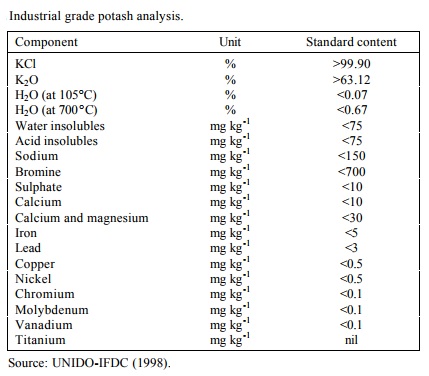
Potassium hydroxide (KOH), also known as caustic potash, is the largestvolume K product for non-fertilizer use. It is produced by the electrolysis of industrial KCl and is widely used for manufacturing soaps, detergents, grease, catalysts, synthetic rubber, matches, dyes and insecticides. Caustic potash is also as a liquid fertilizer and as an ingredient in alkaline batteries and photographic film processing chemicals.
Potassium hydroxide is a raw material in the production of various K salts, mainly K carbonates, and also citrates, silicates, acetates, etc. Potassium carbonate confers excellent clarity to glass thus is used for most fine optical lenses, eyeglasses, fine crystal, glassware, chinaware and TV tubes. Potassium bicarbonate is used largely in the food and pharmaceutical industries.
Potash-derived compounds and salts are also used in the production of metal fluxes, cured meats, tempered steel, paper fumigants, case hardened steel, bleaching agents, baking powder, cream of tartar and beverages. Worldwide, industrial KCl is estimated to be used as follows: detergents and soaps, 30-35%; glass and ceramics, 25-28%; textiles and dyes 20-22%; chemicals and drugs, 13-15%; and other uses, 7-5% (UNIDO-IFDC, 1998). |
| Uses |
- Potassium chloride is a widely used reagent in biochemistry and molecular biology. It is a component of phosphate buffered saline (PBS, Product No. P 3813) and of polymerase chain reaction (PCR) buffer (50 mM KCl).
- KCl is also used in studies of ion transport and potassium channels.
- KCl is also utilized in the solubilization, extraction, purification, and crystallization of proteins.
- The use of KCl in the crystallization of histone core octamers has been reported.
|
| Definition |
ChEBI: A metal chloride salt with a K(+) counterion. |
| Production Methods |
Potassium chloride occurs naturally as the mineral sylvite or sylvine; it also occurs in other minerals such as sylvinite, carnallite, and kainite. Commercially, potassium chloride is obtained by the solar evaporation of brine or by the mining of mineral deposits. |
| Definition |
potassium chloride: A white crystallinesolid, KCl, which is soluble inwater and very slightly soluble inethanol; cubic; r.d. 1.98; m.p. 772°C;sublimes at 1500°C. Potassium chlorideoccurs naturally as the mineralsylvite (KCl) and as carnallite(KCl·MgCl2·6H2O); it is produced industriallyby fractional crystallizationof these deposits or of solutions fromlake brines. It has the interestingproperty of being more soluble thansodium chloride in hot water but lesssoluble in cold. It is used as a fertilizer,in photography, and as a sourceof other potassium salts, such as thechlorate and the hydroxide. It haslow toxicity. |
| Brand name |
Apo-k;Celeka;Durules-k;Kadalex;Kalinorm;Kalipor;Kalium durules;K-long;Miopotasio;Plenish-k;Potasion;Roychlor;Rum-k;Swiss-kal sr;Ultra-k-chlor. |
| World Health Organization (WHO) |
Potassium chloride has been used for many years to correct potassium deficiency. The use of fast-acting tablets has been associated with lesions of the gastro-intestinal mucosa, which have led to their general withdrawal. |
| General Description |
Potassium chloride (KCl) is a water-soluble metal salt that comprises of potassium and chlorine. It can be extracted from minerals and salt water. KCl can be used in industries such as cosmetics, food, biomedical, chemical and fertilizer.
|
| Air & Water Reactions |
Hygroscopic. Water soluble. |
| Reactivity Profile |
Potassium chloride is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture . Reacts with concentrated sulfuric acid to generate fumes of hydrogen chloride. |
| Health Hazard |
Potassium chloride is an essential constituent of the body for intracellular osmotic pressure and buffering, cell permeability, acid-base balance, muscle contraction and nerve function.
SYMPTOMS: Large doses of Potassium chloride usually induce vomiting, so acute intoxication by mouth is rare. If no pre-existing kidney damage, it is rapidly excreted. Poisoning disturbs the rhythm of heart. Large doses by mouth can cause gastrointestinal irritation, purging, weakness, and circulatory disturbances. |
| Fire Hazard |
Flammability data is not available, but Potassium chloride is probably nonflammable. |
| Agricultural Uses |
Muriate of potash or potassium chloride (KCl), is a major potash fertilizer. It is water soluble and is generally blended with other components to make it a multi-nutrient fertilizer. It has a higher salt index than potassium sulphate and is recommended for most crops except tobacco, potato and grapes, which are sensitive to chloride ions. |
| Agricultural Uses |
Potassium chloride (KCl), also known as muriate of potash, is generally blended with other components to make it a multinutrient fertilizer. It is a white crystalline solid, available in fine, coarse and granular grades. It is the least expensive carrier of potassium in the fertilizer market. This important fertilizer contains about 48 to 52% plant food as potassium and about 48% chloride. Coarser potassium blends well with granular N-P compounds to form an NPK-blended multinutrient fertilizer.
At least 78 % of the potassium salts are estimated to be consumed worldwide, in the form of potassium chloride, and over 90% of all processed potassium is used as fertilizer. Muck, peat and sands are generally potassiumdeficient, whereas arid soils are mostly potassium-rich, with 448 kg/ha or more of readily available potassium.
Potassium chloride is neutral and totally watersoluble. It can be applied to all soils and crops that are not sensitive to chlorides. Soluble soil-potassium is adsorbed and retained by soil colloids and thus prevented from leaching. Roots take up potassium in the ionic form.
Potassium chloride is best applied either while sowing or prior to it. However, when soils are light or coarsetextured, the applied potassium may be lost through leaching. So, it is preferable to apply potassium in split doses. On heavy soils, the fertilizer is placed advantageously in bands, as in the case of phosphatic fertilizers.
Potassium chloride is manufactured from potash minerals or brine. Sylvinite, which is a mixture of potassium chloride and halite, is the major potash mineral used for potassium chloride manufacture. A large percentage of potassium chloride is mined and refined either by the floatation or crystallization process. Both processes, of which the floatation process is more common, involve the separation of potassium chloride from sodium chloride. Fine potassium chloride is a freeflowing material which does not cake in dry places. |
| Pharmaceutical Applications |
Potassium chloride is widely used in a variety of parenteral and nonparenteral pharmaceutical formulations. Its primary use, in parenteral and ophthalmic preparations, is to produce isotonic solutions.
Potassium chloride is also used therapeutically in the treatment of hypokalemia.
Many solid-dosage forms of potassium chloride exist including: tablets prepared by direct compression and granulation; effervescent tablets; coated, sustained-release tablets; sustained- release wax matrix tablets;microcapsules;pellets; and osmotic pump formulations.
Experimentally, potassium chloride is frequently used as a model drug in the development of new solid-dosage forms, particularly for sustained-release or modified-release products. Potassium chloride is also used widely in the food industry as a dietary supplement, pH control agent, stabilizer, thickener, and gelling agent. It can also be used in infant formulations. |
| Industrial uses |
Potassium chloride is a colorless or white crystallinecompound of the composition KCl, usedfor molten salt baths for the heat treatment ofsteels. The specific gravity is 1.987. A bathcomposed of three parts potassium chloride andtwo parts barium chloride is used for hardeningcarbon-steel drills and other tools. Steel toolsheated in this bath and quenched in a 3% sulfuricacid solution have a very bright surface.A common bath is made up of potassium chlorideand common salt and can be used for temperaturesup to 900°C.
Potassium chloride is used in the porcelainenamel industry as a setting-up agent in titaniumcover coats. In general, the quantities ofpotassium chloride, when used as an electrolyte,will be approximately the same as sodiumnitrite, which it replaces. However, KCl doesnot aid tearing resistance as does nitrite. Themain advantage in using potassium chloride isthe freedom from yellowing or creaming whenused in a blue-white enamel. Potassium chloridemay exert an adverse effect on the glossand may cause a slight decrease in the acidresistingproperties of the enamel, although thelatter effect is somewhat debatable. |
| Clinical Use |
Hypokalaemia |
| Safety Profile |
A human poison by ingestion. Poison experimentally by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: nausea, blood clotting changes, carhac arrhythmias. An eye irritant. Mutation data reported. Explosive reaction with BrF3; sulfuric acid + potassium permanganate. When heated to decomposition it emits toxic fumes of K2O and Cl-. |
| Safety |
Potassium chloride is used in a large number of pharmaceutical formulations, including oral, parenteral, and topical preparations, both as an excipient and as a therapeutic agent.
Potassium ions play an important role in cellular metabolism and imbalances can result in serious clinical effects. Orally ingested potassium chloride is rapidly absorbed from the gastrointestinal tract and excreted by the kidneys. Potassium chloride is more irritant than sodium chloride when adminstered orally, and ingestion of large quantities of potassium chloride can cause effects such as gastrointestinal irritation, nausea, vomiting, and diarrhea.
High localized concentrations of potassium chloride in the gastrointestinal tract can cause ulceration: hence the development of the many enteric-coated and wax matrix sustained-release preparations that are available.Although it is claimed that some formulations cause less ulceration than others, it is often preferred to administer potassium chloride as an aqueous solution. However, solutions have also been associated with problems, mainly due to their unpleasant taste.
Parenterally, rapid injection of strong potassium chloride solutions can cause cardiac arrest; in the adult, solutions should be infused at a rate not greater than 750 mg/hour.
Therapeutically, in adults, up to 10 g orally, in divided doses has been administered daily, while intravenously up to 6 g daily has been used.
(guinea pig, oral): 2.5 g/kg
(mouse, IP): 1.18 g/kg
(mouse, IV): 0.12 g/kg
(mouse, oral): 0.38 g/kg
(rat, IP): 0.66 g/kg
(rat, IV): 0.14 g/kg
(rat, oral): 2.6 g/kg |
| Drug interactions |
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists: increased risk of hyperkalaemia.
Ciclosporin: increased risk of hyperkalaemia.
Potassium-sparing diuretics: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia. |
| Metabolism |
Potassium is excreted mainly by the kidneys; it is secreted in the distal tubules in exchange for sodium or hydrogen ions. Some potassium is excreted in the faeces and small amounts may also be excreted in sweat. |
| storage |
Potassium chloride tablets become increasingly hard on storage at low humidities. However, tablets stored at 76% relative humidity showed no increase or only a slight increase in hardness.The addition of lubricants, such as 2% w/w magnesium stearate, reduces tablet hardness and hardness on aging.Aqueous potassium chloride solutions may be sterilized by autoclaving or by filtration.
Potassium chloride is stable and should be stored in a well-closed container in a cool, dry place. |
| Purification Methods |
Dissolve it in conductivity water, filter it, and saturate it with chlorine (generated from conc HCl and KMnO4). Excess chlorine is boiled off, and the KCl is precipitated by HCl (generated by dropping conc HCl into conc H2SO4). The precipitate is washed with water, dissolved in conductivity water at 90-95o, and crystallised by cooling to about -5o. The crystals are drained at the centrifuge, dried in a vacuum desiccator at room temperature, then fused in a platinum dish under N2, cooled and stored in a desiccator. Potassium chloride has also been sublimed in a stream of pre-purified N2 gas and collected by electrostatic discharge [Craig & McIntosh Can J Chem 30 448 1952]. |
| Incompatibilities |
Potassium chloride reacts violently with bromine trifluoride and with a mixture of sulfuric acid and potassium permanganate. The presence of hydrochloric acid, sodium chloride, and magnesium chloride decreases the solubility of potassium chloride in water. Aqueous solutions of potassium chloride form precipitates with lead and silver salts.
Intravenous aqueous potassium chloride solutions are incompatible with protein hydrolysate. |
| Regulatory Status |
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections, ophthalmic preparations, oral capsules, and tablets). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| References |
#
#
//study.com/academy/lesson/what-is-potassium-chloride-uses-formula-side-effects.html |
| |
| Potassium chloride Preparation Products And Raw materials |
|
|
|
|
|
|
|
|
|
|
|
|
|
