Superiority:
PRODUCT DETAILS
Propyl gallate
Product name: propyl gallate
Alias: propyl gallate; propyl 3,4,5-trihydroxybenzoate; propyl gallate; n-propyl 3,4,5-trihydroxybenzoate;
Chemical name: Propyl 3,4,5-trihydroxybenzoate
Molecular formula: C 10 H 12 O 5
Molecular weight: 212.21
Structural formula:
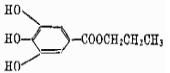
Property: White to milky white crystalline particles, odorless, slightly bitter. Insoluble in water, slightly soluble in cottonseed oil, peanut oil and lard. Propyl gallate is relatively stable, and will react with metal ions such as copper and iron, turning it into purple or dark green.
Specifications: Comply with national standards GB 1886.14, GBT 26441; British Pharmacopoeia; American Pharmacopoeia; European Pharmacopoeia; chemical reagent standards; American food standards.
Technical index:
|
Appearance
|
White crystal or light white powder
|
|
content
|
98%-102%
|
|
Melting point range
|
146-150℃
|
|
Loss on drying
|
≤0.5%
|
|
Residue on ignition
|
≤0.1%
|
|
Other Index
|
See COA
|
Main Uses: As antioxidants in fats, oil-containing foods and pharmaceutical preparations. It is an oil-soluble antioxidant that is allowed to be used in my country and widely used abroad. Its antioxidant capacity for lard is stronger than BHA or BHT, and its antioxidant effect will be enhanced when it is mixed with BHA and BHT.
|
Dosage of propyl gallate in food ( W %)
|
|
Animal oil 0.001-0.01
|
Vegetable oil 0.001-0.02
|
|
Whole milk powder 0.005-0.01
|
Margarine 0.001-0.01
|
|
Bread 0.001-0.004
|
Cereals 0.003
|
|
Chewing gum base 0.1
|
Candy 0.01
|
Storage: Avoid moisture and light, sealed tightly, no contact with metal.
Product validity: two years.
Packing : 25KG/cardboard drum (¢350×500). It can be packaged according to customer requirements, such as carton, woven bag, and then pallet.
| |
| Propyl gallate Chemical Properties |
| Hazard Codes |
Xn |
| Risk Statements |
22-43-36/37/38 |
| Safety Statements |
24-37-36-26 |
| WGK Germany |
2 |
| RTECS |
LW8400000 |
| TSCA |
Yes |
| HS Code |
29182950 |
| Hazardous Substances Data |
121-79-9(Hazardous Substances Data) |
| Toxicity |
LD50 in mice, rats, hamsters, rabbits (g/kg): 1.70-3.50, 2.1-7, 2.48, 2.75 orally; LD50 i.p. in rats: 0.38 g/kg (J. Am. Coll. Toxicol.) |
| |
| Propyl gallate Usage And Synthesis |
| Description |
Propyl gallate (also known as propyl 3, 4, 5-trihydroxybenoate) is a kind of ester formed through the condensation of gallic acid and propanol. It appears as a fine white to creamy-white crystalline powder. It has long been used as a kind of antioxidants to be supplied to foods especially animal fats and vegetable oil, being especially effective with polyunsaturated fats. Propyl gallate, as an anti-oxidant, can protect the food and oils from the attack of hydrogen peroxide and oxygen free radicals, having an effect similar to the superoxide dismutase. It can also be applied to ethers, emulsion, waxes, and transformer oil as the antioxidants. |
| References |
#
#
//www.hmdb.ca/metabolites/HMDB33835 |
| Description |
It caused contact dermatitis in a baker and in a female confectioner who fried doughnuts, primarily sensitized by her night cream; the margarine probably contained gallates. |
| Description |
Propyl gallate is an antioxidant with antimicrobial activity. It is hepatoprotective in vitro and in vivo, preventing CCl4 induced lipoperoxidation and reduction in polysomes in rat liver. Propyl gallate (100 mg/kg, i.p.) increases expression of HIF-1α, EPO, and VEGF mRNA levels and the number of normal neurons in rat brains after 8 minutes of forebrain ischemia. Propyl gallate in combination with potassium sorbate is bactericidal and bacteriostatic against S. aureus strains known to produce enterotoxins in food. Propyl gallate is commonly added to foods to prevent autoxidation and microbial growth. |
| Chemical Properties |
Propyl gallate is an odorless powder having a slightly bitter taste. It functions particularly well in stabilizing animal fats and vegetable oils. With a melting point of 148°C, propyl gallate loses its effectiveness during heat processing and is therefore not suitable in frying applications that involve temperatures exceeding 190°C. Propyl gallate chelates iron ions and forms an unappealing, blue–black complex. Hence, it is always used with chelators such as citric acid to eliminate the pro-oxidative iron and copper catalysts. Good synergism is obtained with BHA and BHT; however, application with TBHQ is not permitted. For additional details, refer to Burdock (1997). |
| Chemical Properties |
white to light beige crystalline powder |
| Chemical Properties |
Propyl gallate is a white, odorless or almost odorless crystalline powder, with a bitter astringent taste that is not normally noticeable at the concentrations employed as an antioxidant. |
| Uses |
Propyl Gallate is an antioxidant that is the n-propylester of 3,4,5-tri- hydroxybenzoic acid. natural occurrence of propyl gallate has not been reported. it is commercially prepared by esterification of gallic acid with propyl alcohol followed by distillation to remove excess alcohol. |
| Uses |
propyl gallate is an anti-oxidant with preservative properties. |
| Uses |
Propyl Gallate is a known inhibitor of Tyrosinase, a polyphenol oxidase, which is an important enzyme in pigment biosynthesis in various organisms. It has also recently been seen to boost biodiesel li pid biosynthesis in cultures. |
| Uses |
Antioxidant for cosmetics, foods, fats, oils, ethers, emulsions, waxes, transformer oils. |
| Preparation |
Produced commercially by the esterification of gallic acid with propyl alcohol followed by distillation to remove the excess alcohol. |
| Production Methods |
Propyl gallate is prepared by the esterification of 3,4,5-trihydroxybenzoic acid (gallic acid) with n-propanol. Other alkyl gallates are prepared similarly using an appropriate alcohol of the desired alkyl chain length. |
| General Description |
Fine white to creamy-white crystalline powder. Odorless or with a faint odor. Melting point 150°C. Insoluble in water. Slightly bitter taste. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Propyl gallate can react with oxidizing agents. Incompatible with strong acids, strong bases and strong reducing agents. Darkens in the presence of iron and iron salts. Contact with metals should be avoided . |
| Hazard |
Use in foods restricted to 0.02% of fat con- tent. |
| Fire Hazard |
Propyl gallate is combustible. |
| Pharmaceutical Applications |
Propyl gallate has become widely used as an antioxidant in cosmetics, perfumes, foods, and pharmaceuticals since its use in preventing autoxidation of oils was first described in 1943.It is primarily used, in concentrations up to 0.1% w/v, to prevent the rancidity of oils and fats;it may also be used at concentrations of 0.002% w/v to prevent peroxide formation in ether, and at 0.01% w/v to prevent the oxidation of paraldehyde. Synergistic effects with other antioxidants such as butylated hydroxyanisole and butylated hydroxytoluene have been reported. Propyl gallate is also said to possess some antimicrobial properties;
Studies have shown that, when added to powder blends containing ketorolac, propyl gallate significantly increases the drug stability in the preparation.
Other alkyl gallates are also used as antioxidants and have approximately equivalent antioxidant properties when used in equimolar concentration; however, solubilities vary;
Propyl gallate has also been investigated for its therapeutic properties, mainly in animal studies. |
| Contact allergens |
This gallate ester (E 311) is an antioxidant frequently used in the food, cosmetic, and pharmaceutical industries to prevent the oxidation of unsaturated fatty acids into rancid-smelling compounds. It causes cosmetic dermatitis mainly from lipsticks and induced contact dermatitis in a baker, and in a female confectioner, primarily sensitized by her night cream, who fried doughnuts the margarine probably containing gallates. |
| Biochem/physiol Actions |
An antioxidant that exhibits antimicrobial activity. Propyl gallate has been reported to be an effective antioxidant-based hepatoprotector, both in vitro and in vivo. It has also been shown to prevent neuronal apoptosis and block the death of neurons exposed to FeSO4/GA as well as partially protect endothelial cells against TNF-induced apoptosis. |
| Toxicology |
Propyl gallate (n-propyl-3,4,5-trihydroxybenzoate) is used in vegetable oils and butter. When 1.2 or 2.3% propyl gallate was added to feed for rats, loss of weight was observed. This may be due to the rats reluctance to eat food that was contaminated with the bitter taste of propyl gallate. When it was given for 10 to 16 months at the 2 to 3% level, 40% of the rats died within the first month and the remainder showed severe growth inhibition. Autopsies of rats indicated kidney damage resulting from the ingestion of propyl gallate. However, no other animal studies show serious problems and further studies indicated that propyl gallate does not cause serious chronic toxicities. |
| Safety Profile |
Poison by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Safety |
It has been reported, following animal studies, that propyl gallate has a strong contact sensitization potential.Propyl gallate has also produced cytogenic effects in CHO-K1 cells.However, despite this, there have been few reports of adverse reactions to propyl gallate.Those that have been described include contact dermatitis, allergic contact dermatitis,and methemoglobinemia in neonates.
The WHO has set an estimated acceptable daily intake for propyl gallate at up to 1.4 mg/kg body-weight.
(cat, oral): 0.4 g/kg
(mouse, oral): 1.7 g/kg
(rat, oral): 2.1 g/kg
(rat, IP): 0.38 g/kg |
| storage |
Propyl gallate is unstable at high temperatures and is rapidly destroyed in oils that are used for frying purposes.
The bulk material should be stored in a well-closed, nonmetallic container, protected from light, in a cool, dry place. |
| Purification Methods |
Crystallise the ester from aqueous EtOH or *C6H6 (m 146-146.5o). [Beilstein 10 III 2078, 10 IV 2003.] |
| Incompatibilities |
The alkyl gallates are incompatible with metals, e.g. sodium, potassium, and iron, forming intensely colored complexes. Complex formation may be prevented, under some circumstances, by the addition of a sequestering agent, typically citric acid. Propyl gallate may also react with oxidizing materials. |
| Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM injections; oral, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| |
| Propyl gallate Preparation Products And Raw materials |
|
| Tag:Propyl gallate(121-79-9) Related Product Information |
|
|
|
|
|
About Our Group
Leader Biochemical Group is a large leader incorporated industry manufacturers and suppliers of advanced refined raw materials From the year of 1996 when our factory was put into production to year of 2020, our group has successively invested in more than 52 factories with shares and subordinates.We focus on manufacture Pharm & chemicals, functional active ingredients, nutritional Ingredients, health care products, cosmetics, pharmaceutical and refined feed, oil, natural plant ingredients industries to provide top quality of GMP standards products.All the invested factories' product lines cover API and intermediates, vitamins, amino acids, plant extracts, daily chemical products, cosmetics raw materials, nutrition and health care products, food additives, feed additives, essential oil products, fine chemical products and agricultural chemical raw materials And flavors and fragrances. Especially in the field of vitamins, amino acids, pharmaceutical raw materials and cosmetic raw materials, we have more than 20 years of production and sales experience. All products meet the requirements of high international export standards and have been recognized by customers all over the world. Our manufacture basement & R&D center located in National Aerospace Economic & Technical Development Zone Xi`an Shaanxi China. Now not only relying on self-cultivation and development as well as maintains good cooperative relations with many famous research institutes and universities in China. Now, we have closely cooperation with Shanghai Institute of Organic Chemistry of Chinese Academy of Science, Beijing Institute of Material Medical of Chinese Academy of Medical Science, China Pharmaceutical University, Zhejiang University. Closely cooperation with them not only integrating Science and technology resources, but also increasing the R&D speed and improving our R&D power. Offering Powerful Tech supporting Platform for group development. Keep serve the manufacture and the market as the R&D central task, focus on the technical research. Now there are 3 technology R & D platforms including biological extract, microorganism fermentation and chemical synthesis, and can independently research and develop kinds of difficult APIs and pharmaceutical intermediates. With the strong support of China State Institute of Pharmaceutical Industry (hereinafter short for CSIPI), earlier known as Shanghai Institute of Pharmaceutical Industry (SIPI), we have unique advantages in the R & D and industrialization of high-grade, precision and advanced products. Now our Group technical force is abundant, existing staff more that 1000 people, senior professional and technical staff accounted for more than 50% of the total number of employees, including 15 PhD research and development personnel, 5 master′ S degree in technical and management personnel 9 people. We have advanced equipment like fermentation equipment and technology also extraction, isolation, purification, synthesis with rich production experience and strict quality control system, According to the GMP required, quickly transforming the R&D results to industrial production in time, it is our advantages and our products are exported to North and South America, Europe, Middle East, Africa, and other five continents and scale the forefront in the nation, won good international reputation. We believe only good quality can bring good cooperation, quality is our key spirit during our production, we are warmly welcome clients and partner from all over the world contact us for everlasting cooperation, Leader will be your strong, sincere and reliable partner in China.
Our Group profiles
Our Factories production lines



Our Factories R&D ability


Our Factories warehouse


Details:
Product information
Propyl gallate
Product name: propyl gallate
Alias: propyl gallate; propyl 3,4,5-trihydroxybenzoate; propyl gallate; n-propyl 3,4,5-trihydroxybenzoate;
Chemical name: Propyl 3,4,5-trihydroxybenzoate
Molecular formula: C 10 H 12 O 5
Molecular weight: 212.21
Structural formula:

Property: White to milky white crystalline particles, odorless, slightly bitter. Insoluble in water, slightly soluble in cottonseed oil, peanut oil and lard. Propyl gallate is relatively stable, and will react with metal ions such as copper and iron, turning it into purple or dark green.
Specifications: Comply with national standards GB 1886.14, GBT 26441; British Pharmacopoeia; American Pharmacopoeia; European Pharmacopoeia; chemical reagent standards; American food standards.
Technical index:
|
Appearance
|
White crystal or light white powder
|
|
content
|
98%-102%
|
|
Melting point range
|
146-150℃
|
|
Loss on drying
|
≤0.5%
|
|
Residue on ignition
|
≤0.1%
|
|
Other Index
|
See COA
|
Main Uses: As antioxidants in fats, oil-containing foods and pharmaceutical preparations. It is an oil-soluble antioxidant that is allowed to be used in my country and widely used abroad. Its antioxidant capacity for lard is stronger than BHA or BHT, and its antioxidant effect will be enhanced when it is mixed with BHA and BHT.
|
Dosage of propyl gallate in food ( W %)
|
|
Animal oil 0.001-0.01
|
Vegetable oil 0.001-0.02
|
|
Whole milk powder 0.005-0.01
|
Margarine 0.001-0.01
|
|
Bread 0.001-0.004
|
Cereals 0.003
|
|
Chewing gum base 0.1
|
Candy 0.01
|
Storage: Avoid moisture and light, sealed tightly, no contact with metal.
Product validity: two years.
Packing : 25KG/cardboard drum (¢350×500). It can be packaged according to customer requirements, such as carton, woven bag, and then pallet.
| |
| Propyl gallate Chemical Properties |
| Hazard Codes |
Xn |
| Risk Statements |
22-43-36/37/38 |
| Safety Statements |
24-37-36-26 |
| WGK Germany |
2 |
| RTECS |
LW8400000 |
| TSCA |
Yes |
| HS Code |
29182950 |
| Hazardous Substances Data |
121-79-9(Hazardous Substances Data) |
| Toxicity |
LD50 in mice, rats, hamsters, rabbits (g/kg): 1.70-3.50, 2.1-7, 2.48, 2.75 orally; LD50 i.p. in rats: 0.38 g/kg (J. Am. Coll. Toxicol.) |
| |
| Propyl gallate Usage And Synthesis |
| Description |
Propyl gallate (also known as propyl 3, 4, 5-trihydroxybenoate) is a kind of ester formed through the condensation of gallic acid and propanol. It appears as a fine white to creamy-white crystalline powder. It has long been used as a kind of antioxidants to be supplied to foods especially animal fats and vegetable oil, being especially effective with polyunsaturated fats. Propyl gallate, as an anti-oxidant, can protect the food and oils from the attack of hydrogen peroxide and oxygen free radicals, having an effect similar to the superoxide dismutase. It can also be applied to ethers, emulsion, waxes, and transformer oil as the antioxidants. |
| References |
#
#
# |
| Description |
It caused contact dermatitis in a baker and in a female confectioner who fried doughnuts, primarily sensitized by her night cream; the margarine probably contained gallates. |
| Description |
Propyl gallate is an antioxidant with antimicrobial activity. It is hepatoprotective in vitro and in vivo, preventing CCl4 induced lipoperoxidation and reduction in polysomes in rat liver. Propyl gallate (100 mg/kg, i.p.) increases expression of HIF-1α, EPO, and VEGF mRNA levels and the number of normal neurons in rat brains after 8 minutes of forebrain ischemia. Propyl gallate in combination with potassium sorbate is bactericidal and bacteriostatic against S. aureus strains known to produce enterotoxins in food. Propyl gallate is commonly added to foods to prevent autoxidation and microbial growth. |
| Chemical Properties |
Propyl gallate is an odorless powder having a slightly bitter taste. It functions particularly well in stabilizing animal fats and vegetable oils. With a melting point of 148°C, propyl gallate loses its effectiveness during heat processing and is therefore not suitable in frying applications that involve temperatures exceeding 190°C. Propyl gallate chelates iron ions and forms an unappealing, blue–black complex. Hence, it is always used with chelators such as citric acid to eliminate the pro-oxidative iron and copper catalysts. Good synergism is obtained with BHA and BHT; however, application with TBHQ is not permitted. For additional details, refer to Burdock (1997). |
| Chemical Properties |
white to light beige crystalline powder |
| Chemical Properties |
Propyl gallate is a white, odorless or almost odorless crystalline powder, with a bitter astringent taste that is not normally noticeable at the concentrations employed as an antioxidant. |
| Uses |
Propyl Gallate is an antioxidant that is the n-propylester of 3,4,5-tri- hydroxybenzoic acid. natural occurrence of propyl gallate has not been reported. it is commercially prepared by esterification of gallic acid with propyl alcohol followed by distillation to remove excess alcohol. |
| Uses |
propyl gallate is an anti-oxidant with preservative properties. |
| Uses |
Propyl Gallate is a known inhibitor of Tyrosinase, a polyphenol oxidase, which is an important enzyme in pigment biosynthesis in various organisms. It has also recently been seen to boost biodiesel li pid biosynthesis in cultures. |
| Uses |
Antioxidant for cosmetics, foods, fats, oils, ethers, emulsions, waxes, transformer oils. |
| Preparation |
Produced commercially by the esterification of gallic acid with propyl alcohol followed by distillation to remove the excess alcohol. |
| Production Methods |
Propyl gallate is prepared by the esterification of 3,4,5-trihydroxybenzoic acid (gallic acid) with n-propanol. Other alkyl gallates are prepared similarly using an appropriate alcohol of the desired alkyl chain length. |
| General Description |
Fine white to creamy-white crystalline powder. Odorless or with a faint odor. Melting point 150°C. Insoluble in water. Slightly bitter taste. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Propyl gallate can react with oxidizing agents. Incompatible with strong acids, strong bases and strong reducing agents. Darkens in the presence of iron and iron salts. Contact with metals should be avoided . |
| Hazard |
Use in foods restricted to 0.02% of fat con- tent. |
| Fire Hazard |
Propyl gallate is combustible. |
| Pharmaceutical Applications |
Propyl gallate has become widely used as an antioxidant in cosmetics, perfumes, foods, and pharmaceuticals since its use in preventing autoxidation of oils was first described in 1943.It is primarily used, in concentrations up to 0.1% w/v, to prevent the rancidity of oils and fats;it may also be used at concentrations of 0.002% w/v to prevent peroxide formation in ether, and at 0.01% w/v to prevent the oxidation of paraldehyde. Synergistic effects with other antioxidants such as butylated hydroxyanisole and butylated hydroxytoluene have been reported. Propyl gallate is also said to possess some antimicrobial properties;
Studies have shown that, when added to powder blends containing ketorolac, propyl gallate significantly increases the drug stability in the preparation.
Other alkyl gallates are also used as antioxidants and have approximately equivalent antioxidant properties when used in equimolar concentration; however, solubilities vary;
Propyl gallate has also been investigated for its therapeutic properties, mainly in animal studies. |
| Contact allergens |
This gallate ester (E 311) is an antioxidant frequently used in the food, cosmetic, and pharmaceutical industries to prevent the oxidation of unsaturated fatty acids into rancid-smelling compounds. It causes cosmetic dermatitis mainly from lipsticks and induced contact dermatitis in a baker, and in a female confectioner, primarily sensitized by her night cream, who fried doughnuts the margarine probably containing gallates. |
| Biochem/physiol Actions |
An antioxidant that exhibits antimicrobial activity. Propyl gallate has been reported to be an effective antioxidant-based hepatoprotector, both in vitro and in vivo. It has also been shown to prevent neuronal apoptosis and block the death of neurons exposed to FeSO4/GA as well as partially protect endothelial cells against TNF-induced apoptosis. |
| Toxicology |
Propyl gallate (n-propyl-3,4,5-trihydroxybenzoate) is used in vegetable oils and butter. When 1.2 or 2.3% propyl gallate was added to feed for rats, loss of weight was observed. This may be due to the rats reluctance to eat food that was contaminated with the bitter taste of propyl gallate. When it was given for 10 to 16 months at the 2 to 3% level, 40% of the rats died within the first month and the remainder showed severe growth inhibition. Autopsies of rats indicated kidney damage resulting from the ingestion of propyl gallate. However, no other animal studies show serious problems and further studies indicated that propyl gallate does not cause serious chronic toxicities. |
| Safety Profile |
Poison by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Safety |
It has been reported, following animal studies, that propyl gallate has a strong contact sensitization potential.Propyl gallate has also produced cytogenic effects in CHO-K1 cells.However, despite this, there have been few reports of adverse reactions to propyl gallate.Those that have been described include contact dermatitis, allergic contact dermatitis,and methemoglobinemia in neonates.
The WHO has set an estimated acceptable daily intake for propyl gallate at up to 1.4 mg/kg body-weight.
(cat, oral): 0.4 g/kg
(mouse, oral): 1.7 g/kg
(rat, oral): 2.1 g/kg
(rat, IP): 0.38 g/kg |
| storage |
Propyl gallate is unstable at high temperatures and is rapidly destroyed in oils that are used for frying purposes.
The bulk material should be stored in a well-closed, nonmetallic container, protected from light, in a cool, dry place. |
| Purification Methods |
Crystallise the ester from aqueous EtOH or *C6H6 (m 146-146.5o). [Beilstein 10 III 2078, 10 IV 2003.] |
| Incompatibilities |
The alkyl gallates are incompatible with metals, e.g. sodium, potassium, and iron, forming intensely colored complexes. Complex formation may be prevented, under some circumstances, by the addition of a sequestering agent, typically citric acid. Propyl gallate may also react with oxidizing materials. |
| Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM injections; oral, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| |
| Propyl gallate Preparation Products And Raw materials |
|
| Tag:Propyl gallate(121-79-9) Related Product Information |
|
