| Product Name: |
BISMUTH SUBSALICYLATE |
| Synonyms: |
Bismuth subsalicylate, USP XVI-PH.FrX° Edition;Bismuth hyposalicylate;Bismuth (Ⅲ)Subsalicylate;Bismuth subsalicylate usp34;C07870;Bismuth Subsalicylate (100 mg);BisMuth(III) subsalicylate 99.9% trace Metals basis;Bismuth subsalicylat |
| CAS: |
14882-18-9 |
| MF: |
C7H5BiO4 |
| MW: |
362.09 |
| EINECS: |
238-953-1 |
| Product Categories: |
Organometallics;HALINONE |
| Mol File: |
14882-18-9.mol |
 |
| |
| BISMUTH SUBSALICYLATE Chemical Properties |
| Melting point |
>350 °C(lit.) |
| storage temp. |
Sealed in dry,Room Temperature |
| solubility |
Practically insoluble in water and in alcohol. It dissolves in mineral acids with decomposition. |
| form |
Powder |
| color |
White |
| Water Solubility |
Practically insoluble in water, alcoholSoluble in acid and alkali. Insoluble in cold water, ethanol, diethyl ether and n-octanol. |
| Merck |
14,1285 |
| BRN |
9170211 |
| InChIKey |
ZREIPSZUJIFJNP-UHFFFAOYSA-K |
| EPA Substance Registry System |
Bismuth subsalicylate (14882-18-9) |
| Provider |
Language |
| SigmaAldrich |
English |
| ALFA |
English |
| |
| BISMUTH SUBSALICYLATE Usage And Synthesis |
| Overview |
Bismuth subsalicylate, an active ingredient in the popular medication Pepto-Bismol, is used for the treatment of nausea, heartburn, indigestion, upset stomach, diarrhea, and other temporary discomforts of the stomach and gastrointestinal tract (including duodenal and peptic ulcers, ulcerative colitis, and diarrhea[1–5]. It is also the main ingredient of Kaopectate. It displays anti-inflammatory action (due to salicylic acid) and also can also act as an antacid and mild antibiotic. In conjunction with antibiotic compounds such as tetracycline, clarithromyacin, and amoxicillin, bismuth subsalicylate has been effective in the treatment and eradication of Helicobacter pylori, the bacterium linked to gastritis and ulceration implicated in the development of gastric cancer and gastric lymphoma[6–9].
Bismuth subsalicylate has the empirical chemical formula of C7H5BiO4[10], and it is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi(C6H4(OH)CO2)3). Bismuth subsalicylate is one of the most widely used medicines in this country, estimated to be present in 60% of American households[11]. It has become the medication of choice for the prevention of traveler’s diarrhea[12], is useful for other forms of toxigenic diarrhea[13] and has been a commonly prescribed antidiarrheal medication for non-syndromic episodic diarrhea in both children and adults for nearly a century[14–17]. Although its detailed mode of action is still unknown, it may stimulate fluid and electrolyte absorption and adsorbs bile acids and enterotoxins[18]. In addition to this antidiarrheal activity, bismuth subsalicylate has well-known antibacterial properties[19, 20], Furthermore, because of its salicylate moiety and perhaps because of bismuth as well, bismuth subsalicylate is thought to be anti-inflammatory[13].
BSS is an old drug and as early as in 1857 Dr Chambers reported on its efficacy in the management of several diseases of the digestive tract[21]. It is commonly available in the USA and in several other countries without the need of a prescription and is largely used for a wide pattern of gastrointestinal diseases.
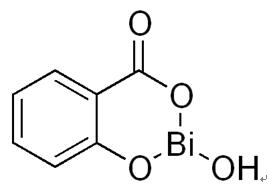
Figure 1. The chemical formula of the bismuth subsalicylate; |
| Bismush salts |
Bismuth salts have been promoted for therapeutic use for over two centuries for a range of clinical conditions including dyspepsia, diarrhoea, syphilis, oral and upper respiratory tract infections, veruccae and warts[22-25]. The bismuth salts or complexes used have also been diverse including such compounds as bismuth subnitrate, bismuth subgallate, bismuth subsalicylate, and bismuth subcarbonate as well as tripotassium dicitrato bismuthate.
The use of the majority of bismuth salts has fallen into dis-favour because of the potential adverse effects, and indeed a number of drug-regulatory authorities are actively delisting bismuth-containing products from medicinal use. Nonetheless, over recent years two bismuth compounds, bismuth subsalicylate and bismuth sub-citrate, have attracted renewed interest because of their newly discovered activities for conditions which respond sub-optimally to existing therapies: travellers’ diarrhoea and peptic ulcer disease, respectively. In travellers’ diarrhoea, effective prophylaxis has been claimed for bismuth subsalicylate[26-29] while in peptic ulcer disease, relapse rates have been shown to be lower following bismuth sub-citrate therapy than following histamine H2antagonist therapy[30-32]. |
| Applications |
Bismuth subsalicylate is used for the treatment of diarrhea, heartburn, colitis, upset stomach and other temporary discomforts of the stomach and gastrointestinal tract in adults and children 12 years of age and older[5, 33]. Bismuth subsalicylate belongs to a class of medications called antidiarrheal agents. It works by decreasing the flow of fluids and electrolytes into the bowel, reduces inflammation within the intestine, and may kill the organisms that can cause diarrhea. It shows certain antibacterial activity[19, 20]. |
| Pharmacokinetics |
Bismuth subsalicylate displays anti-inflammatory action[due to salicylic acid] and can also act as an antacid and mild antibiotic. It can cause a black tongue and black stools in some users of the drug, when it combines with trace amounts of sulfur in their saliva and gastrointestinal tract. This discoloration is temporary and harmless[5].
In vitro dissociation data and in vivo animal data has demonstrated that bismuth subsalicylate is largely hydrolyzed in the stomach to bismuth oxychloride and salicylic acid. When reaching the small intestine, nondissociated bismuth subsalicylate can further react with other anions[bicarbonate and phosphate] to form insoluble bismuth salts. In the colon, nondissociated bismuth subsalicylate and other bismuth salts react with hydrogen sulfide to produce bismuth sulfide, a highly insoluble black salt responsible for the darkening of the stools[5]. |
| Mode of action |
As an antidiarrheal, the exact mechanism still remains unclear. Several in vitro studies have shown that the mechanisms of bismuth subsalicylate are related to two distinct effects: an action directed against pathogenic microorganisms[34] and an intestinal antisecretory or proabsorptive effect on ion and water transport. This is clearly demonstrated for aspirin[which is a salycilate compound] and probably related to the inhibition of prostaglandin synthesis[35]. Experimental data are indeed solid and consistent and provide the basis to support the concept that bismuth subsalicylate is an anti-diarrhoea drug. Bismuth subsalicylate may exert its antidiarrheal action not only by stimulating absorption of fluid and electrolytes across the intestinal wall[antisecretory action] but also, when hydrolyzed to salicylic acid, by inhibiting synthesis of a prostaglandin responsible for intestinal inflammation and hypermotility. In addition, bismuth subsalicylate binds toxins produced by Escherichia coli. Both bismuth subsalicylate and the intestinal reaction products, bismuth oxychloride and bismuth hydroxide, are believed to have bactericidal action. As an antacid, bismuth has weak antacid properties[5]. |
| Side effects |
Side effect associated with the bismuth subsalicylate include anxiety, any loss of hearing, confusion, constipation, diarrhea, difficulty in speaking or slurred speech, dizziness or lightheadedness, drowsiness, fast or deep breathing, headache[severe or continuing], increasing sweating, increased thirst, mental depression, muscle spasms, muscle weakness, nausea or vomiting, ringing or buzzing in ears, stomach pain, trembling, uncontrollable flapping movements of the hands[especially in elderly patients] or other uncontrolled body movements vision problems[36]. |
| Precautions |
Before taking bismuth subsalicylate, you should consult your doctor in the following few conditions as detailed below[33]:
- You are allergic to salicylate pain relievers such as aspirin, choline magnesium trisalicylate, choline salicylate[Arthropan], diflunisal[Dolobid], magnesium salicylate[Doan's, others], and salsalate[Argesic, Disalcid, Salgesic]; or any other medication.
- You are taking or plant to take specific prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products. Be sure to talk to your doctor or pharmacist about taking bismuth subsalicylate if you take: anticoagulants['blood thinners'] such as warfarin[Coumadin]; a daily aspirin; or medication for diabetes, arthritis or gout.
- You are taking tetracycline antibiotics such as minocycline[Dynacin, Minocin], demeclocycline[Declomycin], doxycycline[Doryx, Vibramycin], and tetracycline[Sumycin], take them at least 1 hour before or 3 hours after taking bismuth subsalicylate.
- You have ever had an ulcer, bleeding problem, stools that are bloody or blackened, or kidney disease. You have a fever or mucus in your stool. If you will be giving bismuth subsalicylate to a child or teenager, tell the child's doctor if the child has any of the following symptoms before he or she receives the medication: yellowing of the skin or eyes, vomiting, listlessness, drowsiness, aggression, seizures, confusion, weakness, or flu-like symptoms. Also tell the child's doctor if the child has not been drinking normally, has had excessive vomiting or diarrhea, or appears dehydrated.
- You are pregnant or are breast-feeding.
|
| References |
- F. L. Suarez, J. Furne, J. Stiehm, C. Garten, M. D. Levitt, Dig. Dis. Sci. 2000, 45, 1444.
- G. G. Briand, N. Burford, Chem. Rev. 1999, 99, 2601.
- P. J. Sadler, H. Li, H. Sun, Coord. Chem. Rev. 1999, 689.
- R. Iffland, Bismuth, Handbook on Metals in Clinical and Analytical Chemistry[Eds.: H. G. Seiler, A. Sigel, H. Sigel], Marcel Dekker, New York, 1994.
- #
- J. Houghton, J. G. Fox, T. C. Wang, Eur. J. Gastroenterol. Hepatol. 2002, 17, 495.
- S. Marchi, F. Costa, M. Bellini, C. Belcari, M. G. Mumolo, A. Tornar, R. Spisni, E. Torelli, G. Maltinti, Eur. J. Gastroenterol. Hepatol. 2002, 13, 547.
- N. Chiba, Can. J. Gastro. 2000, 14, 885.
- D. Y. Graham, G. M. Lew, D. G. Evans, P. D. Klein, Ann. Intern. Med. 1991, 115, 266.
- Merck Index, 11th Edition, 1299
- Gorbach SL. Bismuth therapy in gastrointestinal diseases. Gastroenterology 1990;99:863–875.
- DuPont HL, Sullivan P, Evans DG, Pickering LK, Evans DJ Jr, Vollet JJ, Ericsson CD, Ackerman PB, Tjoa WS. Prevention of traveler’s diarrhea[emporiatric enteritis]. Prophylactic administration of subsalicylate bismuth. JAMA 1980;243:237–241.
- Ericsson CD, Tannenbaum C, Charles TT. Antisecretory and antiinflammatory properties of bismuth subsalicylate. Rev Infect Dis 1990;12:S16–S20.
- DuPont HL, Sullivan P, Pickering LK, Haynes G, Ackerman PB. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university. Gastroenterology 1977;73:715–718.
- Bierer DW. Bismuth subsalicylate: history, chemistry, and safety. Rev Infect Dis 1990;12:S3–S8.
- Gryboski JD, Kocoshis S. Effect of bismuth subsalicylate on chronic diarrhea in childhood: a preliminary report. Rev Infect Dis 1990;12(Suppl]:S36–S40.
- Figueroa-Quintanilla D, Salazar-Lindo E, Sack RB, Leo´n-Baru´a R, Sarabia-Arce S, Campos-Sa´nchez M, Eyzaguirre-Maccan E. A controlled trial of bismuth subsalicylate in infants with acute watery diarrheal disease. N Engl J Med 1993;328:1653–1658.
- Farris RK, Tapper EJ, Powell DW, Morris SM. Effect of aspirin on normal and cholera toxin–stimulated intestinal electrolyte transport. J Clin Invest 1976;57:916–924.
- Graham DY, Estes MK, Gentry LO. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli–induced diarrhea in volunteers. Gastroenterology 1983;85:1017–1022.
- Manhart MD. In vitro antimicrobial activity of bismuth subsalicylate and other bismuth salts. Rev Infect Dis 1990;12:S11–S15.
- Chambers TK. Lectures on the management of the digestion in disease. Lecture 5 on diarrhea. Lancet 1857; 2: 185–7
- Thomas, C.C.[1937] A clinical evaluation of oral bismuth[bismutrate] therapy in early infectious syphilis in the female, American Journat of Syphilis, Gonorrhoea and Venereal Diseases, 21,513523.
- Dodd, K., Johnston,L .M. & Buddingh, G.H.[1938] Herpetic stornatitis. Journal of Paedzarrics, 12,
- Lipman Cohen, E.[1958] Inefficacy of sodium bismuth triglycollamate in the treatment of warts. British Journal of Dermatology, 70,254-255.
- Konturek, S.J., Radecki,T, Piastucki, I. & Drozdowicz, D.[1986] Advances in the understanding of the mechanism of cytoprotective action by colloidal bismuth subcitrate. Scandinavian Journal of gastroenterology 122,610.
- Albert, K.S., Welch, R.D., Desante, K.A. & Disanto, A.R.[1979] Decreased tetracycline bioavailability caused by a bismuth subsalicylate antidiarrheal mixture. Journal of pharmaceutical Sciences, 68,586-588.
- Anonymous[1980] Salicylate in Pepto-Bismol. MedicaI Letter on Drugs and Therapeurics, 22,63.
- Feldman, S., Chen, S.L., Pickering, L.K., Cleary, T.G., Ericsson, C.D. & Hulse, M.[1981] Salicylate absorption from a bismuth subsalicylate tid diarrheal preparation[Pepto-Bismol]. Clinical Pharmacology and Therapeurics, 29,788-792.
- Du Pont, H.L.[1987] Bismuth subsalicylate in the treatment and prevention of diarrhoea1 disease. Drug Intelligence and Clinical Pharmacy, 21,687-693.
- Marshall, B.J., Goodwin, C.S., Warren, J.R. et @I.[1987] Long term healing of gastritis and low duodenal ulcer relapse after eradication of campylobacter pyloridis: a prospective double-blind study. Gastroenterology, 92, 1518-1521.
- Lee, F.I., Samloff, I.M. & Hardman, M.[1985] Comparison of tri-potassium di-citrato bismuthate tablets with ranitidine in healing and relapse of duodenal ulcers. Lancer, i, 1299-1303.
- Hamilton, I., O'Connor, H.J., Wood, N.C., Bradbury, I. & Axton, A.T.R.[1986] Healing and recurrence of duodenal ulcer after treatment with tripotassium dicitrato bismuthate[TDB] tablets or cimetidine. Gut, 27, 1061 10
- #
- Manhart MD. In vitro antimicrobia l activity of bismuth subsalicylat e and other bismuth salts. Rev Infect Dis 1990; 12: S11–S15
- Gorbach SL. Bismuth therapy in gastrointestina l diseases. Gastroenterolog y 1990; 99: 863–75
- #
|
| Chemical Properties |
White or almost white powder. |
| Uses |
anticoagulant |
| Uses |
Used to treat nausea, heartburn, indigestion, upset stomach, diarrhea, and other temporary discomforts of the stomach and gastrointestinal tract. |
| Uses |
To impart pearly surface to cellulose-base, polystyrene, and phenolformaldehyde resins. Typical tap bulk density 0.43g/cm^3Bismuth subsalicylate is used to treat temporary discomforts of the stomach and gastrointestinal tract, such as diarrhea, indigestion, heartburn and nausea. It also acts as an antacid. It is practiced to decrease the number of bowel movements and to make the stool firmer. It is also used to reduce inflammation within the intestine. It is further used in growth media for selective isolation of microorganisms. |
| Indications |
Bismuth subsalicylate (Pepto-Bismol) also binds intestinal toxins and may coat irritated mucosal surfaces. |
| General Description |
White crystalline powder or fluffy white solid. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
BISMUTH SUBSALICYLATE is stable in air but is sensitive to light. BISMUTH SUBSALICYLATE is unstable in alkaline solutions. BISMUTH SUBSALICYLATE decomposes in boiling water and alkalis into a more basic salt. |
| Hazard |
A poison by ingestion. Human systemic effects. |
| Health Hazard |
SYMPTOMS: Symptoms of exposure to BISMUTH SUBSALICYLATE may include headache, nausea, foul breath, blue-black line on the gums and stomatitis. |
| Fire Hazard |
Flash point data for BISMUTH SUBSALICYLATE are not available, but BISMUTH SUBSALICYLATE is probably combustible. |
| Side effects |
This compound is a salicylate and may therefore produce signs of salicylate toxicity (e.g., ringing of the ears) if taken chronically, especially with aspirin. Bismuth is radiopaque and may interfere with radiological examinations. Its use may cause temporary gray-black discoloration of the stool and brown pigmentation of the tongue. High dose Pepto-Bismol (8 tablets/day) has been efficacious in some patients with diarrhea secondary to collagenous or lymphocytic colitis. |
| Veterinary Drugs and Treatments |
In veterinary medicine, bismuth subsalicylate products are used to treat diarrhea and as a component of “triple therapy” for treating Helicobacter GI infections. The drug is also used in humans for other GI symptoms (indigestion, cramps, gas pains) and in the treatment and prophylaxis of traveler’s diarrhea. |
| |
| BISMUTH SUBSALICYLATE Preparation Products And Raw materials |
|
|
|
|
|
|
