Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
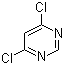
Cas:131860-97-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryJinan Finer Chemical Co., Ltd
Product description: Product name Methyl (E)-2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylate CAS number 131860-97-4 Assay ≥98% Appearance Yellowish powder Capacity 200mt/year
Cas:131860-97-4
Min.Order:10 Gram
FOB Price: $12.0
Type:Lab/Research institutions
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:131860-97-4
Min.Order:1
Negotiable
Type:Other
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1, High quality with competitive price: 1) Standard:BP/USP/EP/Enterprise standard 2) All Purity≥99% 3) We are manufacturer and can provide high quality products with factory price. 2, Fast and safe delivery 1) Parcel can be sent
Cas:131860-97-4
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:131860-97-4
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:131860-97-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:131860-97-4
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Methyl (E)-2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylate Basic information Product Name: Methyl (E)-2-[2-(6-chloropyrimidin-
Cas:131860-97-4
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:131860-97-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Sinotech Import&Export Corporation
Chemical Names: Methyl (E)-2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylate Chemical Structure: Molecular Formula: C15H13ClN2O4 CAS: 13
Cas:131860-97-4
Min.Order:5 Metric Ton
FOB Price: $1.0 / 2.0
Type:Other
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:131860-97-4
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryTAIZHOU ZHENYU BIOTECHNOLOGY CO., LTD
Zhenyu biotech exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, zhenyu biotech is your best choice. pls contact with us freely for getting detailed
Cas:131860-97-4
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:131860-97-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:131860-97-4
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:131860-97-4
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Trading Company
inquiryGIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHubei Taiho Chemical Co.,LTD
TAIHO Unique Advantages:1.We're factory2.Free samples available3.Commodity inspection can be done4.ISO9001,Kosher certifications5.10 years experiences Storage:Store in cool &dry place Package:aluminium foil bag/fiber can/plastic drum Application:comp
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Manufacturers
inquiryXi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryWuxi TAA Chemical Industry Co.,LTD.
1.A strong technical force and advanced processing equipments. The quality of the products has been strictly inspected and all kinds of index have reached or exceeded domestic and international standards.2. Now we have established long-term stable re
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Other
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryWuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:25kg,50kg,180kg,200kg,250kg,1000kg,customization Application:Pharma;Industry;Agricultural;chemical reaserch Tran
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBOC Sciences
We are committed to providing our customers with the best products and services at the most competitive prices.Appearance:powder Storage:Room temperature with sealed well Package:according to the clients requirement Application:Use as primary and sec
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryChangchun Artel lmport and Export trade company
Supply top quality products with a reasonable price Application:api
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHebei Quanhe Biotechnology Co. LTD
1. Timely and efficient service to ensure communication with customers2. Produce products of different specifications and sizes according to your requirements.3. Quality procedures and standards recognized by SGS. Advanced plant equipment ensures sta
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Yuanye Bio-Technology Co., Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:1g Application:Pharma;Industry;Agricultural;chemical reaserch Transportation:by express or by sea Port:Any port
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryChemlyte Solutions
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Other
inquiryNANJING SUNNY PHARMATECH CO., LTD
We are professional supplier in China for this product, we are dedicated to providing customers with the best quality, price, and service. Please feel free to send us inquiries.Appearance:Please contact us for product details Package:As per shipment
Cas:131860-97-4
Min.Order:0
Negotiable
Type:Trading Company
inquirySynthetic route
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dichloropyrimidine; C12H15O5(1-)*Na(1+) With sodium carbonate; methyl 1,4-diazabicyclo<2.2.2>octane-2-carboxylate In toluene at 20℃; for 2h; Stage #2: With zinc(II) chloride In toluene for 6h; Solvent; Reagent/catalyst; Reflux; | 97.3% |

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In acetonitrile at 70℃; for 8h; | 96.9% |
-
-
383428-73-7
3-((α)‐2‐(2‐(6‐chloropyrimidin-4‐yl)oxy)phenyl)methyl-3-methoxyacrylate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With methanesulfonic acid for 1h; Heating; Large scale; | 96% |
| With methanesulfonic acid In toluene at 85℃; for 1.5h; Large scale; |
-
-
484064-87-1
methyl 2-(2-((6-chloropyrimidin-4-yl)oxy)phenyl)acetate

-
-
77-78-1
dimethyl sulfate

-
-
149-73-5
trimethyl orthoformate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-(2-((6-chloropyrimidin-4-yl)oxy)phenyl)acetate; trimethyl orthoformate With titanium tetrachloride; triethylamine In dichloromethane at -5 - 20℃; Inert atmosphere; Stage #2: dimethyl sulfate With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water; toluene at 20 - 50℃; | 91% |

-
-
1193-21-1
4,6-dichloropyrimidine

-
-
114077-42-8
(E)-3-methoxy-2-(2-hydroxyphenyl)-acrylic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 0℃; Inert atmosphere; | 84% |
| With caesium carbonate In N,N-dimethyl-formamide at 0℃; for 4h; Inert atmosphere; | 84.6% |
| With potassium carbonate In N,N-dimethyl-formamide at 0℃; for 12h; Inert atmosphere; | 83.1% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 15h; | 67% |
-
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; sodium hydroxide In toluene at 80 - 90℃; for 3h; | 82.3% |
| With methanesulfonic acid In acetic anhydride at 90℃; for 2h; | 80% |
| With sodium hydrogencarbonate at 160℃; under 20 Torr; for 2h; Product distribution / selectivity; |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 71% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With acetic anhydride In toluene |
-
-
103497-78-5
(E)-3-methoxy-2-(2-benzyloxyphenyl)acrylic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 10% palladium on activated carbon; hydrogen / ethyl acetate / 6 h / 20 °C / 3040.2 Torr 2: caesium carbonate / N,N-dimethyl-formamide / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 2: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen; 5%-palladium/activated carbon / methanol / 18 h / 20 °C / 760.05 Torr 2: potassium carbonate / N,N-dimethyl-formamide / 15 h / 20 °C View Scheme |
-
-
163041-47-2
(Z)-3-methoxy-2-iodo-acrylic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 2: 10% palladium on activated carbon; hydrogen / ethyl acetate / 6 h / 20 °C / 3040.2 Torr 3: caesium carbonate / N,N-dimethyl-formamide / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 2: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 3: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 9.25 h / 20 - 90 °C / Sonication; Inert atmosphere 2: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C 3: caesium carbonate / N,N-dimethyl-formamide / 4 h / 0 °C / Inert atmosphere View Scheme |
-
-
190661-29-1
2-benzyloxyphenylboronic acid

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 2: 10% palladium on activated carbon; hydrogen / ethyl acetate / 6 h / 20 °C / 3040.2 Torr 3: caesium carbonate / N,N-dimethyl-formamide / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 2: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 3: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 9.25 h / 20 - 90 °C / Sonication; Inert atmosphere 2: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C 3: caesium carbonate / N,N-dimethyl-formamide / 4 h / 0 °C / Inert atmosphere View Scheme |
-
-
478413-47-7
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylic acid

-
-
124-41-4
sodium methylate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| In methanol |
-
-
40800-90-6
3-(α-methoxy)methylene benzofuran-2-(3H)-ketone

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium / 0.5 h / -10 °C 1.2: 1 h / Inert atmosphere 2.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 60 °C 3.1: methanesulfonic acid / acetic anhydride / 2 h / 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 1 h / -5 - 0 °C 2: methyl 1,4-diazabicyclo<2.2.2>octane-2-carboxylate; sodium carbonate / toluene / 4 h / 20 °C 3: acetic anhydride; methanesulfonic acid / toluene / 8 h / 95 - 100 °C View Scheme | |
| Multi-step reaction with 3 steps 1: polyethylene glycol 600 / tetrahydrofuran / 5 - 20 °C / Inert atmosphere; Large scale 2: sodium methylate / tetrahydrofuran / 75 h / 25 °C / Large scale 3: methanesulfonic acid / toluene / 1.5 h / 85 °C / Large scale View Scheme |
-
-
175971-61-6
2-(2-hydroxyphenyl)-3,3-dimethoxypropionic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 60 °C 2: methanesulfonic acid / acetic anhydride / 2 h / 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium carbonate / toluene / 4 h / 20 °C 2: methanesulfonic acid; acetic anhydride / 6 h / 105 - 110 °C View Scheme | |
| Multi-step reaction with 2 steps 1: methyl 1,4-diazabicyclo<2.2.2>octane-2-carboxylate; sodium carbonate / toluene / 4 h / 20 °C 2: acetic anhydride; methanesulfonic acid / toluene / 8 h / 95 - 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium carbonate / toluene / 4 h / 20 °C 2: acetic anhydride; methanesulfonic acid / toluene / 6 h / 105 - 110 °C View Scheme |
-
-
614-75-5
2-Hydroxyphenylacetic acid

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 2-Methylpropionic anhydride / 10 h / 100 °C 2.1: sodium / 0.5 h / -10 °C 2.2: 1 h / Inert atmosphere 3.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 60 °C 4.1: methanesulfonic acid / acetic anhydride / 2 h / 90 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: acetyl chloride; hydrogenchloride / 20 h / 20 °C 2.1: potassium carbonate / N,N-dimethyl-formamide / 10 h / 20 °C 3.1: sodium hydride / N,N-dimethyl-formamide / 3 h 3.2: 2 h / 20 °C 4.1: hydrogen; 5%-palladium/activated carbon / methanol / 18 h / 20 °C / 760.05 Torr 5.1: potassium carbonate / N,N-dimethyl-formamide / 15 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: acetic anhydride / 2.5 h / 110 °C / Inert atmosphere; Large scale 2: 21 h / 110 °C / Large scale 3: polyethylene glycol 600 / tetrahydrofuran / 5 - 20 °C / Inert atmosphere; Large scale 4: sodium methylate / tetrahydrofuran / 75 h / 25 °C / Large scale 5: methanesulfonic acid / toluene / 1.5 h / 85 °C / Large scale View Scheme | |
| Multi-step reaction with 3 steps 1.1: acetic anhydride / 2 h / 100 °C / Inert atmosphere; Large scale 1.2: 19 h / 100 °C / Large scale 2.1: tetrahydrofuran; methanol / 72 h / 3 - 24 °C / Inert atmosphere; Large scale 3.1: methanesulfonic acid / 1 h / Heating; Large scale View Scheme |
-
-
5788-17-0
methyl (2E)-3-methoxy-2-propenoate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: iodine; pyridine / tetrachloromethane / 72 h / 0 - 20 °C / Inert atmosphere 2: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 3: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 4: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme |
-
-
31575-75-4
2-bromophenyl benzyl ether

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: magnesium; iodine / tetrahydrofuran / 2 h / 47 °C / Inert atmosphere 1.2: 4 h / -30 - 20 °C / Inert atmosphere 1.3: Grignard Reaction / 0.5 h / 20 °C 2.1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 3.1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 4.1: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: magnesium; iodine / tetrahydrofuran / 0.08 h / 35 - 47 °C / Inert atmosphere 1.2: 5 h / -30 °C / pH 2 2.1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 9.25 h / 20 - 90 °C / Sonication; Inert atmosphere 3.1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C 4.1: caesium carbonate / N,N-dimethyl-formamide / 4 h / 0 °C / Inert atmosphere View Scheme |
-
-
95-56-7
2-hydroxybromobenzene

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 3.5 h / 30 °C / Inert atmosphere 2.1: magnesium; iodine / tetrahydrofuran / 2 h / 47 °C / Inert atmosphere 2.2: 4 h / -30 - 20 °C / Inert atmosphere 2.3: Grignard Reaction / 0.5 h / 20 °C 3.1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 10 h / 90 °C / Inert atmosphere 4.1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C / 760.05 Torr 5.1: potassium carbonate / N,N-dimethyl-formamide / 12 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 3.5 h / 30 °C / Inert atmosphere 2.1: magnesium; iodine / tetrahydrofuran / 0.08 h / 35 - 47 °C / Inert atmosphere 2.2: 5 h / -30 °C / pH 2 3.1: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 9.25 h / 20 - 90 °C / Sonication; Inert atmosphere 4.1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C 5.1: caesium carbonate / N,N-dimethyl-formamide / 4 h / 0 °C / Inert atmosphere View Scheme |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
40800-90-6
3-(α-methoxy)methylene benzofuran-2-(3H)-ketone

-
-
124-41-4
sodium methylate

-
A
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
B
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With Methyl formate; potassium carbonate; 2-methyl-1,4-diazabicyclo [2,2,2]octane In methanol; toluene at 5 - 20℃; for 1.5h; Temperature; Reagent/catalyst; Time; Inert atmosphere; Overall yield = 92.7 %; | |
| With trimethylamine In methanol; toluene at 5℃; for 6h; Catalytic behavior; Temperature; Reagent/catalyst; | |
| With 3-dimethylamino-1-azabicyclo[2.2.2]octane In methanol; toluene at 5 - 15℃; for 2h; |

-
-
553-86-6
benzofuran-2(3H)-one

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydride / N,N-dimethyl-formamide / 2 h / -10 - 0 °C 2: potassium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C 3: sodium methylate / Methyl formate / 10 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 21 h / 110 °C / Large scale 2: polyethylene glycol 600 / tetrahydrofuran / 5 - 20 °C / Inert atmosphere; Large scale 3: sodium methylate / tetrahydrofuran / 75 h / 25 °C / Large scale 4: methanesulfonic acid / toluene / 1.5 h / 85 °C / Large scale View Scheme | |
| Multi-step reaction with 3 steps 1: acetic anhydride / 95 - 100 °C 2: sodium methylate / toluene / 20 - 25 °C 3: toluene-4-sulfonic acid; sodium hydroxide / toluene / 3 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: water / 12 h / 100 °C / Autoclave 2: sodium / 1 h / 0 °C 3: sodium / 1 h / 0 °C View Scheme |
-
-
40800-88-2
3-formylbenzofuran-2(3H)-one

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C 2: sodium methylate / Methyl formate / 10 h / 20 - 25 °C View Scheme |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
40800-90-6
3-(α-methoxy)methylene benzofuran-2-(3H)-ketone

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With sodium methylate In Methyl formate at 20 - 25℃; for 10h; |
-
-
34846-90-7
methyl 3-methoxyprop-2-enoate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine; iodine / tetrachloromethane / 72 h / 0 - 20 °C / Inert atmosphere 2: potassium phosphate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 9.25 h / 20 - 90 °C / Sonication; Inert atmosphere 3: palladium 10% on activated carbon; hydrogen / ethyl acetate / 12 h / 35 °C 4: caesium carbonate / N,N-dimethyl-formamide / 4 h / 0 °C / Inert atmosphere View Scheme |
-
-
22446-37-3
methyl (2-hydroxyphenyl)acetate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 10 h / 20 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 3 h 2.2: 2 h / 20 °C 3.1: hydrogen; 5%-palladium/activated carbon / methanol / 18 h / 20 °C / 760.05 Torr 4.1: potassium carbonate / N,N-dimethyl-formamide / 15 h / 20 °C View Scheme |
-
-
40525-65-3
2-benzyloxyphenylacetic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 3 h 1.2: 2 h / 20 °C 2.1: hydrogen; 5%-palladium/activated carbon / methanol / 18 h / 20 °C / 760.05 Torr 3.1: potassium carbonate / N,N-dimethyl-formamide / 15 h / 20 °C View Scheme |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
40800-90-6
3-(α-methoxy)methylene benzofuran-2-(3H)-ketone

-
A
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
B
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With sodium methylate; potassium carbonate; 2-methyl-1,4-diazabicyclo [2,2,2]octane In methanol; Methyl formate; toluene at 5 - 20℃; for 1.5h; Reagent/catalyst; Temperature; Inert atmosphere; Overall yield = 92.3 %Chromat.; |

-
-
40800-90-6
(E)-3-(α-methoxy)methylenebenzofuran-2(3H)-one

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: methanol / 1 h / -5 - 0 °C 2: sodium carbonate / toluene / 4 h / 20 °C 3: methanesulfonic acid; acetic anhydride / 6 h / 105 - 110 °C View Scheme |
-
-
125808-20-0
methyl 2-(2-hydroxyphenyl)-3-methoxyacrylate

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium methylate / tetrahydrofuran / 75 h / 25 °C / Large scale 2: methanesulfonic acid / toluene / 1.5 h / 85 °C / Large scale View Scheme |
-
-
40800-90-6
3-(α-methoxy)methylene benzofuran-2-(3H)-ketone

-
A
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
B
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1 h / -5 - 0 °C 2: sodium carbonate; methyl 1,4-diazabicyclo<2.2.2>octane-2-carboxylate / toluene / 2 h / 20 °C View Scheme |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| Stage #1: salicylonitrile With potassium carbonate; 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 60℃; for 0.166667h; Stage #2: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate In N,N-dimethyl-formamide at 60 - 84℃; for 0.333333h; Product distribution / selectivity; | 100% |
| Stage #1: salicylonitrile With potassium carbonate In cyclohexanone at 100℃; for 0.166667h; Stage #2: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate; 1,4-diaza-bicyclo[2.2.2]octane In cyclohexanone at 100℃; for 1.66667h; Product distribution / selectivity; | 96.3% |
| Stage #1: salicylonitrile; 1,4-diaza-bicyclo[2.2.2]octane In toluene at 70℃; for 0.5h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 70 - 74℃; for 0.25h; Stage #3: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate In toluene at 70℃; for 0.5h; Product distribution / selectivity; | 94.7% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
611-20-1
salicylonitrile

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| With potassium carbonate; quinuclidine hydrochloride In 4-methyl-2-pentanone at 60 - 80℃; for 0.5h; Product distribution / selectivity; | 100% |
| With potassium carbonate; 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 48 - 65℃; for 1h; Product distribution / selectivity; | 98.3% |
| Stage #1: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate; salicylonitrile In N,N-dimethyl-formamide at 60℃; for 0.0833333h; Stage #2: 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 60℃; for 0.0833333h; Stage #3: With potassium carbonate In N,N-dimethyl-formamide at 60 - 89℃; for 0.166667h; Product distribution / selectivity; | 97.1% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| With potassium carbonate; triethylamine In water; toluene at 80℃; for 10h; | 96.12% |
-
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
611-20-1
salicylonitrile

-
B
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| Stage #1: 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester; (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate; salicylonitrile With potassium carbonate In Isopropyl acetate at 60℃; for 0.166667h; Stage #2: With 1,4-diaza-bicyclo[2.2.2]octane In Isopropyl acetate at 90℃; for 6h; Product distribution / selectivity; | A 91.45% B 4.4% |
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In Isopropyl acetate for 6.5h; Product distribution / selectivity; Heating / reflux; | A 82.85% B 7.05% |
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In cyclohexanone at 90℃; for 2.33333 - 4h; Product distribution / selectivity; | A 73% B 18.3% |

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
1266105-23-0
tert-butyl 6-(3-cyano-4-hydroxyphenyl)hexanoate

-
-
1266105-24-1
tert-butyl 6-(3-cyano-4-(6-(2-((E)-1-(methoxycarbonyl)-2-methoxyvinyl)phenoxy) pyrimidin-4-yloxy)phenyl)hexanoate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 85℃; for 3h; Inert atmosphere; | 91% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
5188-07-8
sodium thiomethoxide

-
A
-
141190-17-2
(E)-methyl 2-[2-(6-(2-cyanoanilino)pyrimidin-4-yloxy)phenyl]-3-methoxypropenoate

-
B
-
131860-99-6
(E)-methyl 2-[2-(6-methylthiopyrimidin-4-yloxy)phenyl]-3-methoxypropenoate

| Conditions | Yield |
|---|---|
| tetrabutylammomium bromide In chloroform; water | A 89% B n/a |

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
41873-65-8
ethyl 2-(2-hydroxyphenyl)acetate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 89% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
108-40-7
toluene-3-thiol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 88% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
137-06-4
2-thiocresol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 85% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
95-48-7
ortho-cresol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 85% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
106-45-6
para-thiocresol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 82% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
119-36-8
methyl salicylate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 82% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
74255-21-3
2-hydroxybenzonitrile sodium salt

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| With silica gel immobilized triethylene diamine catalyst 2 In toluene at 80 - 85℃; for 5h; | 82% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
402-45-9
4-hydroxybenzotrifluoride

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 81% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
108-95-2
phenol

-
-
131860-27-0
(E)-methyl 3-methoxy-2-(2-(6-phenoxypyrimidin-4-yloxy)phenyl)acrylate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 85℃; for 3h; | 80% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
77458-30-1
1-(4-chlorophenyl)-1H-pyrazol-4-ol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 79% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
98-17-9
3-Trifluoromethylphenol

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 76% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
611-20-1
salicylonitrile

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| With potassium carbonate In water | 75.6% |
| With potassium carbonate In water | 70.8% |
| With potassium hydroxide In water | 61.5% |

-
-
143230-42-6
2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
B
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| Stage #1: 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester; (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate; 2-hydroxybenzonitrile potassium salt With potassium carbonate In Isopropyl acetate; water at 50℃; Stage #2: With 1,4-diaza-bicyclo[2.2.2]octane In Isopropyl acetate; water for 3.75 - 5h; Product distribution / selectivity; Heating / reflux; | A 72.6% B 15.4% |
| Stage #1: 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3,3-dimethoxypropionic acid methyl ester; (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In Isopropyl acetate; water at 50℃; Stage #2: 2-hydroxybenzonitrile potassium salt In Isopropyl acetate; water for 4.33333h; Product distribution / selectivity; Heating / reflux; | A 68% B 12.4% |

-
-
6554-61-6
4,5-dichloropyrimidine

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
6636-78-8
2-chloro-3-hydroxypyridine

-
-
133001-57-7
(E)-methyl 2-[2-(6-(2-chloropyrid-3-yloxy)pyrimidin-4-yloxy)phenyl]-3-methoxypropenoate

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper(I) chloride In N,N-dimethyl-formamide | 70% |

-
-
67-56-1
methanol

-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; Inert atmosphere; | 69% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

| Conditions | Yield |
|---|---|
| Stage #1: 3-[(4-hydroxyphenyl)imino]-5-methyl-1-propylindolin-2-one With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 0.5h; Stage #2: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate In N,N-dimethyl-formamide at 90℃; | 69% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View