Hefei TNJ chemical industry co.,ltd
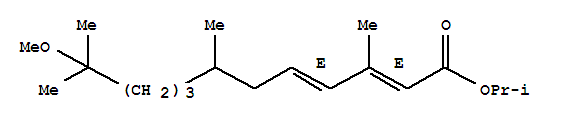
ISO product Name Methoprene Synonyms S-Methoprene Molecular Formula C19H34O3 Molecular Weight 310.4715 InChI InChI=1/C19H34O3/c1-15(2)22-1
Cas:40596-69-8
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:40596-69-8
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
Dayangchem’s R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. Near 20 years DayangChem has provided dif
Cas:40596-69-8
Min.Order:10 Gram
FOB Price: $1.0 / 2.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Hebei yanxi chemical co.,LTD.
CHENGDU YANXI is a comprehensive manufacturer and an international distribution of products throughout the world. Specialized in Scrap metal, Chemical raw materials, Paper products and color industry. We aim to become leading position in global dis
Hebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:40596-69-8
Min.Order:1 Gram
FOB Price: $6.0 / 9.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung, LG, Merck, Thermo Fisher Scientific and so o
Cas:40596-69-8
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:40596-69-8
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:40596-69-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Minstar Chemical Co., Ltd
Synonyms: ALTOSID(R);11-METHOXY-3,7,11-TRIMETHYL-2E,4E-DODECADIENOIC ACID, ISOPROPYL ESTER;METHOPRENE (TM);METHOPRENE;ISOPROPYL (2E,4E)-11-METHOXY-3,7,11-TRIMETHYL-2,4-DODECADIENOATE;PRECOR;ZR-515;2,4-dodecadienoicacid,11-methoxy-3,7,11-trimethyl-,
Cas:40596-69-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
METHOPRENE CAS:40596-69-8 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates,
Cas:40596-69-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:40596-69-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryChangchun Artel lmport and Export trade company
Product Detail Minimum Order Qty. 10 Gram
Cas:40596-69-8
Min.Order:20 Metric Ton
Negotiable
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:40596-69-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryTriumph International Development Limilted
Triumph has the complete production of G- KG - MT service chain,we can make the new technology into productivity quickly in the research and development of new products. Main Service 1.Own made fine chemical products 2.Out
Cas:40596-69-8
Min.Order:100 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:40596-69-8
Min.Order:1 Kilogram
FOB Price: $1.0 / 100000.0
Type:Lab/Research institutions
inquiryHubei Yuanmeng Biological Technology Co., Ltd.
Hubei Yuanmeng Biological Technology Co., Ltd., which is located in Wuhan, China. We are specializing in the exportation of APIs, and plant extracts ect. Our products has been exported to America, Australia, Brazil, the Europe, Middle East and other
Cas:40596-69-8
Min.Order:1 Kilogram
FOB Price: $3.0 / 5.0
Type:Trading Company
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:40596-69-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:40596-69-8
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquirySHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Cas:40596-69-8
Min.Order:100 Gram
FOB Price: $100.0 / 2000.0
Type:Lab/Research institutions
inquiryJiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
EAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
TAIZHOU ZHENYU BIOTECHNOLOGY CO., LTD
Zhenyu biotech exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, zhenyu biotech is your best choice. pls contact with us freely for getting detailed
Cas:40596-69-8
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:40596-69-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:40596-69-8
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Synthetic route
-
-
87416-87-3
5-(6'-methoxy-2',6'-dimethylheptyl)-3-methyl-2,5-dihydrothiophene-2-carboxylic acid

-
A
-
52341-11-4
Isopropyl (2Z,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate

-
B
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 12h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | A n/a B n/a C 20% |

-
-
87416-89-5
5-(6-Methoxy-2,6-dimethyl-heptyl)-3-methyl-2,5-dihydro-thiophene-2-carboxylic acid isopropyl ester

-
A
-
52341-11-4
Isopropyl (2Z,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate

-
B
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 12h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | A n/a B n/a C 20% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 12h; Product distribution; | A n/a B n/a C 90 mg |


-
A
-
52341-11-4
Isopropyl (2Z,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate

-
B
-
40596-69-8
methoprene

-
C
-
41797-08-4, 55290-71-6, 58222-78-9, 58223-32-8, 68218-72-4
isopropyl 3,7,11-trimethyl-2ξ,4E,10-dodecatrienoate

| Conditions | Yield |
|---|---|
| With lithium carbonate; lithium bromide In N,N-dimethyl-formamide for 1h; Heating; Yield given. Title compound not separated from byproducts; | A n/a B 0.18 g C 10% |
| With lithium carbonate; lithium bromide In N,N-dimethyl-formamide for 1h; Heating; Yields of byproduct given; | A n/a B 0.18 g C 10% |


| Conditions | Yield |
|---|---|
| (i) SOCl2, (ii) /BRN= 635639/; Multistep reaction; |
-
-
3613-30-7
7-methoxy-3,7-dimethyloctanal

-
-
50798-35-1
diisopropyl ester of 2-methyl-3-isopropoxycarbonyl-2-propenylphosphonic acid

-
A
-
52341-11-4
Isopropyl (2Z,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate

-
B
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide In benzene other reagents; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
917-54-4
methyllithium

-
-
80957-93-3
Isopropyl (2Z,4E)-3-Acetoxy-11-methoxy-7,11-dimethyl-2,4-dodecadienoate

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With copper(l) iodide 1.) ether, -30 degC, 10 min; 2.) ether, -20 degC, 2h; Yield given. Multistep reaction; |
-
-
917-54-4
methyllithium

-
-
80957-87-5, 80957-88-6
Isopropyl (4E)-3-Diethoxyphosphoryloxy-11-methoxy-7,11-dimethyl-2,4-dodecadienoate

-
A
-
52341-11-4
Isopropyl (2Z,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate

-
B
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With copper(l) iodide 1.) ether, -30 degC, 30 min; 2.) -78 degC, 1h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
103768-96-3
2-(1-Bromo-7-methoxy-3,7-dimethyl-octyl)-3-methyl-cyclopropanecarboxylic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; zinc dibromide 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h; Multistep reaction; |
-
-
616-44-4
3-Methylthiophene

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 2: 77 percent / H2SO4 conc. / 4 h / Heating 3: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 4: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 5: diethyl ether / 0.5 h / 0 °C 6: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 2: 77 percent / H2SO4 conc. / 4 h / Heating 3: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 4: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 5: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
4434-77-9
2-bromo-6-methylhept-5-ene

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 68 percent / 1.) Br / 2.) THF, 2.) 25 deg C, 30 min 2: 73 percent / NaBH4 / methanol / 0.5 h / 25 °C 3: pyridine; diethyl ether / 6 h / Heating 4: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 5: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 6: 77 percent / H2SO4 conc. / 4 h / Heating 7: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 8: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 9: diethyl ether / 0.5 h / 0 °C 10: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 9 steps 1: 68 percent / 1.) Br / 2.) THF, 2.) 25 deg C, 30 min 2: 73 percent / NaBH4 / methanol / 0.5 h / 25 °C 3: pyridine; diethyl ether / 6 h / Heating 4: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 5: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 6: 77 percent / H2SO4 conc. / 4 h / Heating 7: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 8: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 9: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
4234-93-9
2,6-dimethyl-5-hepten-1-ol

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: pyridine; diethyl ether / 6 h / Heating 2: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 3: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 4: 77 percent / H2SO4 conc. / 4 h / Heating 5: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 6: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 7: diethyl ether / 0.5 h / 0 °C 8: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 7 steps 1: pyridine; diethyl ether / 6 h / Heating 2: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 3: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 4: 77 percent / H2SO4 conc. / 4 h / Heating 5: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 6: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 7: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
87416-83-9
1-bromo-2,6-dimethylhept-5-ene

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 2: 77 percent / H2SO4 conc. / 4 h / Heating 3: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 4: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 5: diethyl ether / 0.5 h / 0 °C 6: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 2: 77 percent / H2SO4 conc. / 4 h / Heating 3: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 4: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 5: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
106-72-9
2,6-dimethyl-5-hepten-1-al

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 73 percent / NaBH4 / methanol / 0.5 h / 25 °C 2: pyridine; diethyl ether / 6 h / Heating 3: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 4: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 5: 77 percent / H2SO4 conc. / 4 h / Heating 6: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 7: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 8: diethyl ether / 0.5 h / 0 °C 9: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 73 percent / NaBH4 / methanol / 0.5 h / 25 °C 2: pyridine; diethyl ether / 6 h / Heating 3: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 4: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 5: 77 percent / H2SO4 conc. / 4 h / Heating 6: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 7: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 8: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
87416-84-0
2-(2',6'-dimethylhept-5'-enyl)-4-methylthiophene

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 77 percent / H2SO4 conc. / 4 h / Heating 2: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 3: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 4: diethyl ether / 0.5 h / 0 °C 5: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 77 percent / H2SO4 conc. / 4 h / Heating 2: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 3: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 4: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
87416-85-1
2-(6'-methoxy-2',6'-dimethylheptyl)-4-methylthiophene

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 2: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 3: diethyl ether / 0.5 h / 0 °C 4: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 2: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 3: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
87416-86-2
5-(6'-methoxy-2',6'-dimethylheptyl)-3-methylthiophene-2-carboxylic acid

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 2: diethyl ether / 0.5 h / 0 °C 3: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 2: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
87416-87-3
5-(6'-methoxy-2',6'-dimethylheptyl)-3-methyl-2,5-dihydrothiophene-2-carboxylic acid

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether / 0.5 h / 0 °C 2: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 2: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 3: 77 percent / H2SO4 conc. / 4 h / Heating 4: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 5: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 6: diethyl ether / 0.5 h / 0 °C 7: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 5.84 g / NaBr / dimethylformamide / 5 h / 50 °C 2: 5.8 g / 1.) n-BuLi, TMEDA / 1.) THF, 2.) -15 deg C, 1 h; 20 deg C, 2 h 3: 77 percent / H2SO4 conc. / 4 h / Heating 4: 1.64 g / 1.) n-BuLi / 1.) Et2O, reflux, 1 h, 2.) THF 5: 0.21 g / LiOH / H2O / 8 h / 25 °C / electrochemical reduction 6: m-CPBA / CH2Cl2 / 12 h / 0 °C View Scheme |
-
-
542-08-5
isopropyl acetoacetate

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 2: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 3: 63 percent / p-toluenesulfonic acid / 6 h / Heating 4: 1.) copper(I) iodide / 1.) ether, -30 degC, 10 min; 2.) ether, -20 degC, 2h View Scheme | |
| Multi-step reaction with 4 steps 2: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 3: 1.) NaH / 1.) ether, 0.5h, room temp.; 2.) ether, 8h, reflux 4: 1.) copper(I) iodide / 1.) ether, -30 degC, 30 min; 2.) -78 degC, 1h View Scheme |
-
-
3613-30-7
7-methoxy-3,7-dimethyloctanal

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Et3N / N-(2-hydroxyethyl)-thiazolium bromide / dioxane / 20 h / 95 - 100 °C 2: sodium borohydride / tetrahydrofuran / 1 h / 0 °C 3: pyridine / 24 h / 25 °C 4: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 5: sodium borohydride / propan-2-ol / 4 h / Heating 6: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 7: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme | |
| Multi-step reaction with 4 steps 2: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 3: 63 percent / p-toluenesulfonic acid / 6 h / Heating 4: 1.) copper(I) iodide / 1.) ether, -30 degC, 10 min; 2.) ether, -20 degC, 2h View Scheme | |
| Multi-step reaction with 4 steps 2: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 3: 1.) NaH / 1.) ether, 0.5h, room temp.; 2.) ether, 8h, reflux 4: 1.) copper(I) iodide / 1.) ether, -30 degC, 30 min; 2.) -78 degC, 1h View Scheme |
-
-
55755-06-1
α-Keto-β-Methyl-buttersaeure-isopropylester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Et3N / N-(2-hydroxyethyl)-thiazolium bromide / dioxane / 20 h / 95 - 100 °C 2: sodium borohydride / tetrahydrofuran / 1 h / 0 °C 3: pyridine / 24 h / 25 °C 4: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 5: sodium borohydride / propan-2-ol / 4 h / Heating 6: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 7: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
-
-
300786-34-9
isopropyl 2-hydroxy-3-methylbutyrate

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: MnO2 2: Et3N / N-(2-hydroxyethyl)-thiazolium bromide / dioxane / 20 h / 95 - 100 °C 3: sodium borohydride / tetrahydrofuran / 1 h / 0 °C 4: pyridine / 24 h / 25 °C 5: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 6: sodium borohydride / propan-2-ol / 4 h / Heating 7: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 8: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 63 percent / p-toluenesulfonic acid / 6 h / Heating 2: 1.) copper(I) iodide / 1.) ether, -30 degC, 10 min; 2.) ether, -20 degC, 2h View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) NaH / 1.) ether, 0.5h, room temp.; 2.) ether, 8h, reflux 2: 1.) copper(I) iodide / 1.) ether, -30 degC, 30 min; 2.) -78 degC, 1h View Scheme |

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 2: 63 percent / p-toluenesulfonic acid / 6 h / Heating 3: 1.) copper(I) iodide / 1.) ether, -30 degC, 10 min; 2.) ether, -20 degC, 2h View Scheme | |
| Multi-step reaction with 3 steps 1: 61 percent / 4N HCl / dioxane / 0.25 h / 80 °C 2: 1.) NaH / 1.) ether, 0.5h, room temp.; 2.) ether, 8h, reflux 3: 1.) copper(I) iodide / 1.) ether, -30 degC, 30 min; 2.) -78 degC, 1h View Scheme |
-
-
103768-95-2
2-(1-Hydroxy-7-methoxy-3,7-dimethyl-octyl)-3-methyl-cyclopropanecarboxylic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 2: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
-
-
103768-92-9
2-Hydroxy-11-methoxy-3,7,11-trimethyl-5-oxo-dodecanoic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: pyridine / 24 h / 25 °C 2: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 3: sodium borohydride / propan-2-ol / 4 h / Heating 4: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 5: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
-
-
103768-91-8
11-Methoxy-3,7,11-trimethyl-2,5-dioxo-dodecanoic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: sodium borohydride / tetrahydrofuran / 1 h / 0 °C 2: pyridine / 24 h / 25 °C 3: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 4: sodium borohydride / propan-2-ol / 4 h / Heating 5: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 6: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
-
-
103768-94-1
2-(7-Methoxy-3,7-dimethyl-octanoyl)-3-methyl-cyclopropanecarboxylic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium borohydride / propan-2-ol / 4 h / Heating 2: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 3: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
-
-
103768-93-0
11-Methoxy-3,7,11-trimethyl-5-oxo-2-(toluene-4-sulfonyloxy)-dodecanoic acid isopropyl ester

-
-
40596-69-8
methoprene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium t-butoxid, 18-Crown-6 / benzene / 0.5 h / Ambient temperature 2: sodium borohydride / propan-2-ol / 4 h / Heating 3: PBr3, LiBr, 2,4,6-collidine / 3 h / -40 - 0 °C 4: 1.) ZnBr, 2.) DBU / 1.) ether, -40 deg C to 0 deg C, 2 h, 2.) 50 deg C, 2 h View Scheme |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View





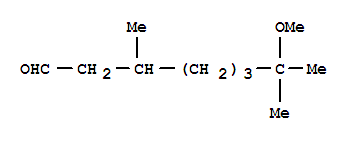


 Xi,
Xi,  N
N