-
Name
1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide
- EINECS 217-579-2
- CAS No. 1892-57-5
- Article Data12
- CAS DataBase
- Density 0.86 g/cm3
- Solubility Soluble in water.
- Melting Point 115 ºC
- Formula C8H17N3
- Boiling Point 197.7 °C at 760 mmHg
- Molecular Weight 155.243
- Flash Point 73.4 °C
- Transport Information UN 2735 8/PG 3
- Appearance COA
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms N-Ethyl-N'-(3-dimethylaminopropyl)carbodiimide;1-Ethyl-3-[3-(dimethylamino)propyl]carbodiimide;1-Ethyl-3-(dimethylaminopropyl)carbodiimide;1-Ethyl-3-(N,N-dimethylamino)propylcarbodiimide;1,3-Propanediamine,N'-(ethylcarbonimidoyl)-N,N-dimethyl- (9CI);Carbodiimide,[3-(dimethylamino)propyl]ethyl- (6CI,7CI,8CI);1-Ethyl-3-(3'-dimethylaminopropyl)carbodiimide;3-(3-Dimethylaminopropyl)-1-ethylcarbodiimide;EDAC;N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide;
- PSA 27.96000
- LogP 1.13190
Synthetic route
-
-
32897-26-0
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With triethylamine; p-toluenesulfonyl chloride In dichloromethane for 4h; Heating; | 60% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With dicarbonyl(cyclopentadienyl)methyliron(II); Triethoxysilane In tetrahydrofuran; 1,3,5-trimethyl-benzene at 60℃; for 24h; Schlenk technique; Inert atmosphere; | 46% |
| With sodium hypochlorite; ethylenediaminetetraacetic acid at 30℃; Reagent/catalyst; Temperature; | |
| With bis(trichloromethyl) carbonate In Petroleum ether at 15 - 45℃; for 3h; Temperature; | 213.6 g |
-
-
542-85-8
Ethyl isothiocyanate

-
-
109-55-7
1-amino-3-(dimethylamino)propane

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| (i) Et2O, (ii) HgO, benzene; Multistep reaction; | |
| (i) Et2O, (ii) HgO, CH2Cl2; Multistep reaction; |
-
-
109-55-7
1-amino-3-(dimethylamino)propane

-
-
109-90-0
ethyl isocyanate

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| (i), (ii) TsCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether / 2 h / Ambient temperature 2: 60 percent / triethylamine, toluenesulfonyl chloride / CH2Cl2 / 4 h / Heating View Scheme |
-
-
25952-53-8
1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With potassium carbonate In trifluoromethylbenzene (BTF); water |
-
-
18997-72-3
N-(3-Dimethylamino-propyl)-dithiocarbamidsaeure

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: chloroformic acid ethyl ester; triethylamine / dichloromethane / 3 h / 10 - 15 °C 2: dichloromethane / 1 h / 10 - 15 °C 3: sodium hypochlorite; ethylenediaminetetraacetic acid / 30 °C View Scheme |
-
-
27421-70-1
3-(dimethylamino)propyl isothiocyanate

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 1 h / 10 - 15 °C 2: sodium hypochlorite; ethylenediaminetetraacetic acid / 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: methanol / 2 h / 10 - 20 °C 2: chloroformic acid ethyl ester; triethylamine / dichloromethane / 3 h / 10 - 15 °C 3: dichloromethane / 1 h / 10 - 15 °C 4: sodium hypochlorite; ethylenediaminetetraacetic acid / 30 °C View Scheme |
-
-
1271-42-7
ferrocenecarboxylic acid

-
-
5680-80-8
methyl (2S)-2-amino-3-hydroxypropanoate hydrochloride

-
-
2592-95-2
benzotriazol-1-ol

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With CuCl In N,N-dimethyl-formamide | 99% |

-
-
36507-55-8
morphine-6-hemisuccinate

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 37℃; for 2h; pH=5.5; | 98% |

-
-
42417-65-2
Z-MeVal-OH

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
80029-43-2, 123333-53-9
1-hydroxybenzotriazol-hydrate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 98% |


-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 2h; pH=4.7 - 4.8; | 98% |
-
-
55147-78-9
bis(bis(trimethylsilyl)amido)tin(II)

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1362855-87-5
[(C2H5NC(N(Si(CH3)3)2)N(CH2)3N(CH3)2)2Sn]

| Conditions | Yield |
|---|---|
| In diethyl ether (Ar); C2H5NCN(CH2)3N(CH3)2 in Et2O added to a soln. of Sn compd. at roomtemp., stirred for 0.5 h; filtered, evapd. (vac.); obtained oily; elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| at 15 - 25℃; for 1h; Large scale; | 95.8% |

| Conditions | Yield |
|---|---|
| at 15 - 25℃; for 1h; Large scale; | 95.5% |
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
38862-25-8
methacrylic acid N-hydroxysuccinimide ester

| Conditions | Yield |
|---|---|
| With sodium sulfate In dichloromethane | 94% |

-
-
2886-33-1
dibenzyl L-aspartate toluene-4-sulphonate

-
-
88950-64-5
1-<(tert-butyloxy)carbonylamino>cyclopropane-1-carboxylic acid

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1415763-45-9
1-[(tert-butoxycarbonyl)amino]cyclopropanecarbonyl-L-aspartic acid dibenzyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine In dichloromethane | 94% |


| Conditions | Yield |
|---|---|
| at 15 - 25℃; for 1h; Large scale; | 94% |
-
-
4530-18-1
2-(tert-butoxycarbonylamino)-3-phenylpropionic acid

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane addn. of 1 equiv. protected phenylalanine ester to Ru-complex, then addn. of Et3N and carbodiimide derivative, stirring at -50°C for 12 h; addn. of water, citric acid and Na2CO3, solvent removal; elem. anal.; | 92% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
13629-22-6
fluoren-9-ylidene-hydrazine

-
-
1445265-19-9
EtNHC(NNfluoren)NHCH2CH2CH2NMe2

| Conditions | Yield |
|---|---|
| With [Ti(pentamethylcyclopentadienyl)(N(xylyl)C4H6N)(tert-butylimido)(tert-butylamine)] In 1,4-dioxane at 80℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 92% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
25952-53-8
1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine hydrochloride In acetonitrile at 15℃; for 1h; Temperature; | 92% |
-
-
677722-99-5, 847675-62-1, 297176-74-0
(Sp)-ortho-(diphenylphosphanyl)ferrocenylcarboxylic acid

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| In dichloromethane (Ar); carbodiimide was added to soln. of Fe complex in CH2Cl2; stirred at room temp. for 20 h; evapd. (vac.); chromd. (silica gel, CH2Cl2/MeOH, 10/1 to 5/1); evapd. (vac.); | 91% |
-
-
55147-78-9
bis(bis(trimethylsilyl)amido)tin(II)

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1362855-81-9
(C2H5NC(N(Si(CH3)3)2)N(CH2)3N(CH3)2)SnN(SiMe3)2

| Conditions | Yield |
|---|---|
| In diethyl ether (Ar); a soln. of C2H5NCN(CH2)3N(CH3)2 added to a soln. of Sn compd. at room temp., stirred for 2 h; filtered, evapd. (vac.); obtained oily; elem. anal.; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; 2,4-dinitrobenzoic acid In dichloromethane at 20℃; for 0.5h; Stage #2: With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; | 91% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 1h; Ambient temperature; | 90% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1362855-85-3
[(C2H5NC(N(Si(CH3)3)2)N(CH2)3N(CH3)2)SnCl]

| Conditions | Yield |
|---|---|
| In diethyl ether (Ar); C2H5NCN(CH2)3N(CH3)2 in Et2O added to a suspn. of Sn compd. at room temp., stirred; filtered, evapd. (vac.); obtained oily; elem. anal.; | 90% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
80-70-6
N,N,N',N'-tetramethylguanidine

-
-
1542158-74-6
C13H30N6

| Conditions | Yield |
|---|---|
| at 120℃; for 0.0833333h; Inert atmosphere; Microwave irradiation; Green chemistry; | 89% |

| Conditions | Yield |
|---|---|
| With [Ti(pentamethylcyclopentadienyl)(N(xylyl)C4H6N)(tert-butylimido)(tert-butylamine)] In toluene at 105℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 87% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
74-88-4
methyl iodide

-
-
138551-31-2
1-[3-(dimethylamino)-propyl]-3-ethylcarbodiimide methiodide

| Conditions | Yield |
|---|---|
| In diethyl ether for 48h; Ambient temperature; | 85% |
| In dichloromethane at 15 - 25℃; for 1h; Large scale; | 81.2% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| In pyridine; trifluoroacetic acid | 83% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
74-88-4
methyl iodide

-
-
22572-40-3
3-{[(ethylimino)methylene]-amino}-N,N,N-trimethyl-1-propanaminium iodide

| Conditions | Yield |
|---|---|
| In dichloromethane at 15 - 25℃; for 1h; Industrial scale; | 81.2% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1415763-66-4
N-allyl-N-(3-{5-[(4-amidinophenoxy)carbonyl]-3-methylthiophen-2-yl}propanoyl)glycine trifluoroacetate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In pyridine | 81% |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
66761-27-1
4-carboxy-3-hydroxyphenyl azide

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1293988-98-3
[2-(4-azido-2-hydroxy-benzoylamino)-ethyl]-carbamicacid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-carboxy-3-hydroxyphenyl azide; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide With benzotriazol-1-ol In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: N-BOC-1,2-diaminoethane In N,N-dimethyl-formamide at 20℃; for 18h; Inert atmosphere; | 75% |

-
-
52833-94-0
2-amino-5-bromonicotinicacid

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-5-bromonicotinicacid; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide With benzotriazol-1-ol In tetrahydrofuran at 0℃; for 0.25h; Stage #2: With isopropylamine In tetrahydrofuran at 20℃; | 75% |
-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1415763-54-0
N-allyl-N-({2-[(2,2,2-trichloroethoxy)carbonyl]-4,5-dihydrothieno[2,3-c]pyridin-6(7H)-yl}acetyl)-L-glutamic acid di-tert-butyl ester

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In pyridine | 74% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| With pyridine; trifluoroacetic acid | 73% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

-
-
1415763-00-6
N-allyl-N-[N-allyl-N-(3-{5-[(4-amidinophenoxy)carbonyl]-3-methylthiophen-2-yl}propanoyl)glycyl]-L-glutamic acid trifluoroacetate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In pyridine | 73% |

-
-
1892-57-5
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide

| Conditions | Yield |
|---|---|
| In chloroform for 1.5h; | 72% |

1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Chemical Properties
The MF of 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide(1892-57-5): C8H17N3
The MW of 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide(1892-57-5): 155.24
EINECS: 217-579-2
bp: 66-68°C 1mm
density: 0.877 g/mL at 20 °C(lit.)
refractive index: n20/D 1.461
Fp: 66-68°C/1mm
storage temp.: -20 °C
Sensitive: Air Sensitive
Vapour Pressure: 0.373 mmHg at 25°C
Categories: straight chain compounds
Synonyms: 1-ETHYL-3-(3-DIMETHYLAMINOPROPYL) CARBODIIMIDE;1-(3-DIMETHYLAMINOPROPYL)-3-ETHYLCARBODIIMIDE;EDAC;EDCI;WATER-SOLUBLE CARBODIIMIDE;WSCD;WSCI;N-ETHYL-N'-(3-DIMETHYLAMINOPROPYL)CARBODIIMIDE
The Structure of 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide(1892-57-5):
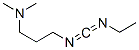
1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Safety Profile
 C
C Risk Statements: 34
34: Causes burns
Safety Statements: 26-36/37/39-45
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
RIDADR: UN 2735 8/PG 3
WGK Germany: 3
F: 1-3-10
F1 Sensitive to air and humidity.
F 3 Hygroscopic.
F 10 Keep under argon.
HazardClass: 8
PackingGroup: III
Related Products
- 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide methiodide
- 189264-03-7
- 189264-44-6
- 189264-45-7
- 189264-47-9
- 18927-05-4
- 189271-66-7
- 189274-36-0
- 189275-74-9
- 189278-27-1
- 189279-58-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View