-
Name
1,1,2,2-Tetrabromoethane
- EINECS 201-191-5
- CAS No. 79-27-6
- Article Data15
- CAS DataBase
- Density 3.004 g/cm3
- Solubility water: 0.63 g/L (20 °C)
- Melting Point 1 °C
- Formula C2H2Br4
- Boiling Point 243.5 °C at 760 mmHg
- Molecular Weight 345.654
- Flash Point 97 °C
- Transport Information UN 2504 6.1/PG 3
- Appearance yellowish heavy liquid
- Safety 24-27-45-61-1/2
- Risk Codes 26-36-52/53
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, T
T
- Synonyms 1,1,2,2-Tetrabromoethylene;Acetylene tetrabromide;Muthmann's liquid;NSC406889;TBE;
- PSA 0.00000
- LogP 3.21820
Synthetic route

| Conditions | Yield |
|---|---|
| With pentafluorosulfanyl bromide at 20℃; for 24h; Irradiation; | A n/a B n/a C 97.1% |


| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; bromine at 105 - 110℃; |

| Conditions | Yield |
|---|---|
| With water; bromine | |
| With water; bromine; potassium bromide at 22℃; Electrolysis.unter langsamem Hindurchleiten von Acetylen; |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide |
-
-
79-01-6
Trichloroethylene

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
594-78-5
1,1,2-tribromo-1,2,2-trichloro-ethane

| Conditions | Yield |
|---|---|
| With bromine Irradiation; |

| Conditions | Yield |
|---|---|
| dibenzoyl peroxide at 100℃; for 2h; Rate constant; Product distribution; other temperature, other time; |

| Conditions | Yield |
|---|---|
| dibenzoyl peroxide at 100℃; for 2h; Rate constant; Product distribution; other temperature; |
-
-
5721-88-0
2-phenyl-1,3-oxathiolane

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
81110-22-7
S-(2-bromoethyl) benzothioate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With Bromoform; di-tert-butyl peroxide at 130℃; for 4.5h; Yields of byproduct given; | A n/a B 16 % Turnov. C n/a |

-
-
27001-65-6
2-propyl-1,3-oxathiolan

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
83124-63-4
2-bromoethyl thiobutyrate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With Bromoform; di-tert-butyl peroxide at 130℃; for 4.5h; Yields of byproduct given; | A n/a B 14 % Turnov. C n/a |

-
-
16048-04-7
2-ethyl-[1,3]oxathiolane

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
83124-62-3
2-bromoethyl thiopropionate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With Bromoform; di-tert-butyl peroxide at 130℃; for 4.5h; Yields of byproduct given; | A n/a B 18 % Turnov. C n/a |


| Conditions | Yield |
|---|---|
| dibenzoyl peroxide at 100℃; for 2h; Rate constant; Product distribution; other temperature; |

| Conditions | Yield |
|---|---|
| dibenzoyl peroxide at 100℃; for 2h; Rate constant; Product distribution; other temperature; |
-
-
75-25-2
Bromoform

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
83124-62-3
2-bromoethyl thiopropionate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With 2-ethyl-[1,3]oxathiolane; di-tert-butyl peroxide at 130℃; for 4.5h; | A n/a B 18 % Turnov. C n/a |

-
-
75-25-2
Bromoform

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
83124-63-4
2-bromoethyl thiobutyrate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With 2-propyl-1,3-oxathiolan; di-tert-butyl peroxide at 130℃; for 4.5h; | A n/a B 14 % Turnov. C n/a |

-
-
75-25-2
Bromoform

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
81110-22-7
S-(2-bromoethyl) benzothioate

-
C
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With 2-phenyl-1,3-oxathiolane; di-tert-butyl peroxide at 130℃; for 4.5h; | A n/a B 16 % Turnov. C n/a |

-
-
75-62-7
Bromotrichloromethane

-
-
83498-70-8
2-(hexyloxy)-1,3-dioxolane

-
-
79-27-6
1,1,2,2-tetrabromoethane

| Conditions | Yield |
|---|---|
| dibenzoyl peroxide at 90℃; for 2h; Rate constant; Product distribution; other temperature; |
-
-
75-25-2
Bromoform

-
-
18816-24-5
7,7-dimethyl-1,4,5,6-tetraphenyl-2,3-benzo-7-silanorbornadiene

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
4095-10-7
dimethyldibromosilane

-
C
-
751-38-2
1,2,3,4-tetraphenylnaphthalene

-
D
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| In hexane at 22.9℃; Rate constant; Product distribution; Mechanism; Irradiation; laser pulse photolysis technique; investigated by UV and 1H NMR measurements; |

-
-
74-84-0
ethane

-
A
-
79-27-6
1,1,2,2-tetrabromoethane

-
B
-
74-96-4
ethyl bromide

-
C
-
106-93-4
ethylene dibromide

| Conditions | Yield |
|---|---|
| With 2AlBr3*CBr4; bromine at 55 - 65℃; under 760 Torr; for 3h; Title compound not separated from byproducts; | A 53 % Turnov. B 3 % Turnov. C 17 % Turnov. |


| Conditions | Yield |
|---|---|
| unterhalb des Siedepunktes; |

| Conditions | Yield |
|---|---|
| im Sonnenlicht; bei manchen Versuchen auch Prod.5: 1.2-Dibrom-1.2-dirhodan-aethan; cis-trans-mixture; |


| Conditions | Yield |
|---|---|
| With iron In N,N-dimethyl-formamide | 93% |
| With ethanol; zinc | |
| With aluminium amalgam |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Katamin AB In water at 25 - 30℃; for 2h; | 90% |
| With diethyl ether; sodium ethanolate | |
| With diethyl ether; sodium |
-
-
3469-69-0
4-iodopyrazole

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1073267-96-5
1,1,2,2-tetrakis(4-iodo-1H-pyrazol-1-yl)ethane

| Conditions | Yield |
|---|---|
| Stage #1: 4-iodopyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 0.5h; Stage #2: 1,1,2,2-tetrabromoethane In dimethyl sulfoxide for 2h; | 86.7% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
683-08-9
Diethyl methylphosphonate

-
A
-
74-96-4
ethyl bromide

-
B
-
598-16-3
1,1,2-tribromoethylene

-
C
-
1832-53-7
ethyl methylphosphonic acid

| Conditions | Yield |
|---|---|
| at 180℃; for 10h; | A 15% B 79% C 37% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
96700-97-9
t-butyldimethylsiloxy-3,5-dimethoxybenzene-4-lithio

-
-
96701-00-7
t-butyldimethylsiloxy-4-bromo-3,5-dimethoxybenzene

| Conditions | Yield |
|---|---|
| In toluene Ambient temperature; | 77% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

| Conditions | Yield |
|---|---|
| With s-C4H9Li In diethyl ether; cyclohexane (Ar); using Schlenk techniques; addn. dropwise s-BuLi/C6H12 to CpFeC5H4CH2C4H7NCH2OMe/Et2O at -78°C; stirring for 1.5 h at -78°C and 1.5 h at -30°C; addn. of C2H2Br4/Et2O at -78°C for 0.5 h; stirring for 0.5 h and 16h at r.t.; quenching(H2O); sepn.; extn. aq. phase with CH2Cl2; washing of aq. phases with aq. Na2S2O5 and brine; extn. of org. phase with aq. citric acid; extn. of cobined aq. phase with Et2O; addn. NaOH to pH=9 at 0°C; extn. with Et2O; drying; chromy.Al2O3; | 74% |

| Conditions | Yield |
|---|---|
| With chlorine monofluoride | 72% |
| With mercury(II) fluoride at 150 - 160℃; | |
| With hydrogen fluoride; mercury(II) oxide at 40 - 50℃; | |
| With silver(II) fluoride at 80℃; Substitution; |

| Conditions | Yield |
|---|---|
| Stage #1: NH-pyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 1h; Stage #2: 1,1,2,2-tetrabromoethane In dimethyl sulfoxide for 5h; | 71.4% |
| Stage #1: NH-pyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 0.5h; Stage #2: 1,1,2,2-tetrabromoethane In dimethyl sulfoxide at 80℃; for 5h; Further stages.; | 57% |

| Conditions | Yield |
|---|---|
| With H2O In diethyl ether 1,1,2,2-tetrabromoethane in Et2O was added slowly to suspn. FcLi2(TMEDA) in Et2O at -60°C, soln. was allowed to warm to room temp., stired for 10 h and hydrolyzed with H2O and stired for 10 min; organic layer was separated and evapd., residue was extd. with Et2O andfiltered, solvent was removed and residue was crystd. from MeOH; | 71% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
A
-
359-19-3
1,1-dibromo-2,2-difluoroethane

-
B
-
598-67-4
1,1,2-tribromo-2-fluoroethane

| Conditions | Yield |
|---|---|
| With chlorine monofluoride | A 12% B 70% |
| With bromine; antimony(III) fluoride at 120 - 125℃; for 23h; | A 19% B 11% |
| With bromine; antimony(III) fluoride at 120 - 125℃; for 23h; |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
238413-86-0
1,2-dibromoferrocene

-
-
1145715-22-5
1,2,3-tribromoferrocene

| Conditions | Yield |
|---|---|
| With lithium tetramethylpiperidinide In tetrahydrofuran; hexane Schlenk techniques used under N2 or Ar, THF soln. of 2 (8.99 mmol) addedto freshly prepd. (from hexane soln. of nBuLi (8.96 mmol) and TMP (8.96 mmol) in THF) soln. of LiTMP and stirred for 3 h at -30°C, C2H2B r4 (8.96 mmol) added at -70°C; allowed to warm to room temp. for 18 h, H2O added, extracted with Et2O, dried over MgSO4, filtered, solvent removed under vac., petroleum ether added, filtered through alumina plug, partial evaporated, cooled to 4°C; XRD; | 69% |

| Conditions | Yield |
|---|---|
| Stage #1: ferrocene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In hexane Inert atmosphere; Stage #2: 1,1,2,2-tetrabromoethane In diethyl ether; hexane at -78℃; for 4h; | 67% |
| Stage #1: ferrocene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine Stage #2: 1,1,2,2-tetrabromoethane at -78℃; |

| Conditions | Yield |
|---|---|
| at 105℃; for 36h; complexation; | 65% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1271-86-9
N,N-dimethylaminomethylferrocene

-
-
245740-46-9, 12110-60-0, 245740-45-8
rac-2-(N,N-dimethylaminomethyl)bromoferrocene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; diethyl ether all manipulations under inert atm. (Ar or N2); soln. of Fe compd. in Et2O cooled to 0°C, Li compd. added slowly, stirred at ambient temp.for 24 h, THF added, cooled to -78°C, Br compd. added, allowed t o warm to room temp., stirred at; ambient temp. overnight, water added, aq. phase extd. with ether, combined org. phase dried with anhydr. MgSO4, filtered, concd., chromy. (alumina, Et2O/Et2NH 95:5); elem. anal.; | 62% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1273-73-0
bromoferrocene

-
-
238413-86-0
1,2-dibromoferrocene

-
-
1145715-22-5
1,2,3-tribromoferrocene

-
-
1145715-26-9
1,2,3,4,5-pentabromoferrocene

| Conditions | Yield |
|---|---|
| With lithium tetramethylpiperidinide In tetrahydrofuran Schlenk techniques used under N2 or Ar, mixt.(10.8 mmol) of 1 (51%), 2 (24%), 4 (18%) and 5 (7%) in THF added to LiTMP (100 mmol) in THF and stirred for 5 h at -30°C, C2H2Br4 (100 mmol) added at -78°C, warmed to room temp. for >16 h; quenched with H2O, extracted with CH2Cl2, organic layer washed with aq. 1M HCl and H2O, dried over MgSO4, solvent removed under vac., chromd. (neutral alumina, hexane), concentrated, allowed to stand at 5°C for 16 h; elem.anal., XRD; | 57% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
13637-65-5
chlorogermane

-
-
917-54-4
methyllithium

-
-
68196-77-0
digermylacetylene

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: LiBr, CH4, MeBr; absence of air and moisture; treatment of C2H2Br4 with 4 equiv. of MeLi (room temp., 1 h), evapn. (vac., 1 h), condensation of 2 equiv. of ClGeH3, warming to room temp. (5 min); fractional low-temp. distn. (condensing at 206 K); | 50% |

-
-
288-13-1
NH-pyrazole

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
C
-
1313194-68-1
(Z)-1,2-bis(1H-pyrazol-1-yl)ethylene

| Conditions | Yield |
|---|---|
| Stage #1: NH-pyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 0.5h; Stage #2: 1,1,2,2-tetrabromoethane In dimethyl sulfoxide at 80℃; for 24h; | A 47% B n/a C n/a |

-
-
512-56-1
trimethyl phosphite

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
A
-
813-78-5
dimethylphosphoric acid

-
B
-
598-16-3
1,1,2-tribromoethylene

| Conditions | Yield |
|---|---|
| at 180℃; for 20h; | A 31% B 45% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1145715-22-5
1,2,3-tribromoferrocene

-
B
-
1145715-26-9
1,2,3,4,5-pentabromoferrocene

| Conditions | Yield |
|---|---|
| With lithium tetramethylpiperidinide In tetrahydrofuran; hexane Schlenk techniques used under N2 or Ar, THF soln. of 4 (6.34 mmol) addedto freshly prepd. (from hexane soln. of nBuLi (15.8 mmol) and TMP (15.8 mmol) in THF) soln. of LiTMP and stirred for 3 h at -30°C, C2H2B r4 (15.8 mmol) added at -70°C; alloed to warm to room temp. for 18 h, H2O added, extracted with Et2O, dried over MgSO4, filtered, solvent removed under vac., recrystd. from petroleum ether/Et2O at 4°C; elem. anal.; | A 44% B 18% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1362991-84-1
2-bromo-3,4,5-triferrocenylthiophene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane (under Ar, Schlenk); Fe-complex dissolved in THF, cooled to -40°C, Li-salt in hexane added dropwise, warmed to -25°C for 35 min, cooled to -40°C, ligand added, warmed to ambient temp., stirred for 3 h; volatiles removed, alumina column chromy. with hexane:toluene 3:1, collected, solvent evapd., crystd. from n-hexane/toluene 5:1; elem. anal.; | 40% |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1293-65-8
1,1'-dibromoferrocene

-
A
-
102-54-5
ferrocene

-
B
-
238413-86-0
1,2-dibromoferrocene

| Conditions | Yield |
|---|---|
| With tetramethylpiperidine; n-butyl lithium In tetrahydrofuran; hexane Schlenk techniques used under N2 or Ar, THF soln. of (BrC5H4)2Fe (25.0 mmol) treated at -70°C with soln. of nBuLi (25.0 mmol), after 30 min TMP (25.0 mmol) added, stirred for 3 h at -30--40°C, C2H2Br4 (25.0 mmol) added at -70°C; allowed to warm to ambient temp. for >18 h, H2O added, aq. phase separated, extracted with Et2O, dried over MgSO4, evaporated, chromd. (neutral alumina, hexane), FcH removed by sublimation; NMR; | A n/a B 36% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1309387-42-5
5-bromo-2,9-di(undecan-6-yl)anthra[2,1,9-def:6,5,10-d'e'f']diisoquinoline-1,3,8,10(2H,9H)-tetraone

-
-
1610377-74-6
helical PDI

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate; potassium hydrogencarbonate In 1,4-dioxane; isopropyl alcohol at 110℃; for 10h; Schlenk technique; Inert atmosphere; Sealed tube; | 30% |
-
-
288-13-1
NH-pyrazole

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
C
-
1313194-68-1
(Z)-1,2-bis(1H-pyrazol-1-yl)ethylene

-
D
-
1313194-72-7
1,1-bis(pyrazol-1-yl)-2-bromoethene

| Conditions | Yield |
|---|---|
| Stage #1: NH-pyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 0.5h; Stage #2: 1,1,2,2-tetrabromoethane In dimethyl sulfoxide at 80℃; for 24h; | A 22% B n/a C n/a D n/a |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1273-73-0
bromoferrocene

-
-
238413-86-0
1,2-dibromoferrocene

-
A
-
1145715-22-5
1,2,3-tribromoferrocene

| Conditions | Yield |
|---|---|
| With lithium tetramethylpiperidinide In tetrahydrofuran Schlenk techniques used under N2 or Ar, THF soln. of mixt. (12.0 mmol) of 1 (52%) and 2 (48%) added to LiTMP (6.00 mmol) in THF and stirred for 4 h at -30°C, C2H2Br4 (6.09 mmol) added at -78°C, allowed to warm to room temp. for >16 h; quenched with H2O, extracted with CH2Cl2, organic layer washed with H2O,dried over MgSO4, solvent removed under vac., chromd. (neutral alumina, hexane); not isolated, detected by NMR; | A 18% B 7% |

-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
1273-73-0
bromoferrocene

-
A
-
238413-86-0
1,2-dibromoferrocene

-
B
-
1145715-26-9
1,2,3,4,5-pentabromoferrocene

| Conditions | Yield |
|---|---|
| With lithium tetramethylpiperidinide In tetrahydrofuran Schlenk techniques used under N2 or Ar, THF soln. of 1 (4.76 mmol) addedto LiTMP (47.5 mmol) in THF and stirred for 5 h at -30°C, C2H2Br 4 (47.5 mmol) added at -78°C, allowed to warm to room temp. for >16 h; quenched with H2O, extracted with CH2Cl2, organic layer washed with aq. 1M HCl and H2O, dried over MgSO4, solvent removed under vac., chromd. (neutral alumina, hexane), concentrated, allowed to stand at 5°C for 16 h; elem.anal., XRD; | A n/a B 11% |


| Conditions | Yield |
|---|---|
| With diethyl ether Irradiation.mit Sonnenlicht; |
-
-
79-27-6
1,1,2,2-tetrabromoethane

-
-
60-29-7
diethyl ether

-
-
141-52-6
sodium ethanolate

-
-
598-16-3
1,1,2-tribromoethylene

1,1,2,2-Tetrabromoethane Chemical Properties
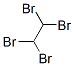
IUPAC: 1,1,2,2-Tetrabromoethane
CAS:79-27-6
The Molecular formula of Acetylene tetrabromide (79-27-6):C2H2Br4
The Molecular Weight of Acetylene tetrabromide (79-27-6):345.65
EINECS:201-191-5
Synonyms: Ethanetetrabromide ; 1,1,2,2-Tetrabromoethane ; Acetylene tetrabromide ; muthmanns liquid ; Tetrabromoethane ; Sym-tetrabromoethane ; Tbe ; 1,1,2,2-Tetrabromaethan
Density:2.967 g/mL at 25oC(lit.)
Melting Point:1oC
Boiling Point:244oC
Flash Point:97oC
Water Solubility:0.63 g/L (20oC)
Stability:Stable. Incompatible with strong oxidizing agents, aluminium, magnesium, alkali metals.
Vapor density:11.9 (vs air)
Vapor pressure:0.1 mm Hg ( 20oC)
Refractive index:n20/D 1.637(lit.)
Appearance:Pale-yellow liquid with a pungent odor similar to camphor or iodoform.
Enthalpy of Vaporization:46.11 kJ/mol
Product Categories:Intermediates of Dyes and Pigments;Organics
Mol File:79-27-6.mol
1,1,2,2-Tetrabromoethane Uses
Acetylene tetrabromide (79-27-6) is a separating mineral by specific gravity. It is used as a solvent for fats, oils, and waxes.
1,1,2,2-Tetrabromoethane Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | AIHAAP American Industrial Hygiene Association Journal. 24 (1963),28. | ||
| 2. | eye-rbt 100 mg MLD | AIHAAP American Industrial Hygiene Association Journal. 24 (1963),28. | ||
| 3. | mmo-sat 10 µg/plate | TECSDY Toxicological and Environmental Chemistry. 15 (1987),101. | ||
| 4. | dnr-esc 29,640 µg/disc | MUREAV Mutation Research. 41 (1976),61. | ||
| 5. | skn-mus TDLo:130 g/kg/74W-I:NEO | JJIND8 JNCI, Journal of the National Cancer Institute. 63 (1979),1433. | ||
| 6. | orl-rat LD50:1200 mg/kg | VRDEA5 Vrachebnoe Delo. Medical Profession(3),(1967),80. | ||
| 7. | ihl-rat LC50:549 mg/m3/4H | 85GMAT Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure Izmerov, N.F., et al.,Moscow, USSR.: Centre of International Projects, GKNT,1982,107. | ||
| 8. | skn-rat LD50:5250 mg/kg | 85GMAT Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure Izmerov, N.F., et al.,Moscow, USSR.: Centre of International Projects, GKNT,1982,107. | ||
| 9. | orl-mus LD50:269 mg/kg | 85GMAT Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure Izmerov, N.F., et al.,Moscow, USSR.: Centre of International Projects, GKNT,1982,107. |
1,1,2,2-Tetrabromoethane Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
1,1,2,2-Tetrabromoethane Safety Profile
Poison by inhalation, ingestion, and intraperitoneal routes. An eye and skin irritant and a narcotic. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. When heated it emits highly toxic fumes of carbonyl bromide and Br−. See also ACETYLENE COMPOUNDS and ALKYNES and BROMIDES.
Safty informations about Acetylene tetrabromide (79-27-6):
Hazard Codes:T+.gif)
Risk Statements:26-36-52/53
26(Very Toxic by inhalation)
36(Irritating to the eyes)
52/53(Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment)
Safety Statements:24-27-45-61-1/2
24(Avoid contact with skin)
27(Take off immediately all contaminated clothing)
45(In case of accident or if you feel unwell, seek medical advice immediately (show label where possible))
61(Avoid release to the environment. Refer to special instructions safety data sheet)
1/2(Keep locked up and out of the reach of children)
RIDADR:UN 2504 6.1/PG 3
WGK Germany:2
RTECS:KI8225000
HazardClass:6.1
HS Code:29033036
1,1,2,2-Tetrabromoethane Standards and Recommendations
OSHA PEL: TWA 1 ppm
ACGIH TLV: TWA 1 ppm
DFG MAK: 1 ppm (14 mg/m3)
1,1,2,2-Tetrabromoethane Analytical Methods
For occupational chemical analysis use NIOSH: see 1,1,2,2-Tetrabromoethane, 2003.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 79276-19-0
- 79277-27-3
- 79277-67-1
- 79286-74-1
- 79286-79-6
- 792899-38-8
- 792911-66-1
- 792924-09-5
- 792942-45-1
- 79295-15-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View