-
Name
1,1-DIMETHYLUREA
- EINECS 209-957-0
- CAS No. 598-94-7
- Article Data60
- CAS DataBase
- Density 1.023 g/cm3
- Solubility soluble in water
- Melting Point 178-183 °C(lit.)
- Formula C3H8N2O
- Boiling Point 130.4 °C at 760 mmHg
- Molecular Weight 88.1093
- Flash Point 32.7 °C
- Transport Information
- Appearance White to off-white crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Urea,1,1-dimethyl- (7CI,8CI);1,1-Dimethylurea;N,N-Dimethylcarbamide;N,N-Dimethylurea;NSC 33603;asym-Dimethylurea;
- PSA 46.33000
- LogP 0.32700
Synthetic route

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With [OsCl2(η6-p-cymene)(PMe2OH)]; water at 40℃; for 0.5h; Catalytic behavior; Mechanism; Reagent/catalyst; Temperature; Concentration; Inert atmosphere; Sealed tube; | 92% |
| With potassium hydroxide | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 12h; Inert atmosphere; | 91% |
| With para-dodecylbenzenesulfonic acid In neat (no solvent) at 80℃; for 0.75h; Green chemistry; | 83% |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 2h; Inert atmosphere; Schlenk technique; | A 88% B 75% |


| Conditions | Yield |
|---|---|
| With cholin chloride ZnCl2; iron oxide at 130℃; for 6h; Green chemistry; | 73% |
| With Iron(III) nitrate nonahydrate In toluene for 16h; Reflux; |
-
-
76952-35-7
2-aminopyrazine-3-carboxamide oxime

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
34859-38-6
2-Cyano-3-<(dimethylaminomethylene)amino>pyrazine

| Conditions | Yield |
|---|---|
| at 75℃; for 0.0833333h; | A 12% B 66% |
| at 75℃; for 0.0833333h; | A 12% B 66% |

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
112085-02-6
2-(benzoylamino)pyridine-3-carboxamide oxime

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
112084-93-2
2-(benzoylamino)-3-cyanopyridine

| Conditions | Yield |
|---|---|
| In chloroform for 2h; Product distribution; Heating; | A 7% B 66% |

-
-
94703-39-6
2,3,3,3-tetrachloro-2-<amino>propionitrile

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate for 1h; Heating; | 39% |
-
-
76952-35-7
2-aminopyrazine-3-carboxamide oxime

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
81411-61-2
3-formylamino-1H-pyrazolo(3,4-b)pyrazine

-
C
-
34859-38-6
2-Cyano-3-<(dimethylaminomethylene)amino>pyrazine

| Conditions | Yield |
|---|---|
| In toluene for 3h; Heating; | A 8% B 36% C 14% |
| In toluene for 6h; Product distribution; Heating; other times and temp.; | A 10% B 35% C 28% |

-
-
106932-14-3
N,N-dimethyl N'-(dimethyl-4,6 pyridyl-2)-2 phenyl-1 vinyluree

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| hydrolysis; | 11% |
-
-
61713-36-8
N,N,O-trimethyl-isourea

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With water |

| Conditions | Yield |
|---|---|
| photochemische Zersetzung; |
-
-
16703-45-0
O-methyl N,N-dimethylthiocarbamate

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| With methanol; ammonia at 100℃; |

| Conditions | Yield |
|---|---|
| beim Stehen; |

| Conditions | Yield |
|---|---|
| at 130 - 140℃; | |
| With water at 130 - 140℃; | |
| With ethanol at 130 - 140℃; |

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| at 100℃; | |
| at 120℃; |

| Conditions | Yield |
|---|---|
| With water at 100℃; | |
| With ethanol at 120℃; | |
| at 100℃; | |
| at 120℃; |

| Conditions | Yield |
|---|---|
| at 160 - 170℃; |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| (i) POCl3, (ii) NH2OH; Multistep reaction; |
-
-
108-47-4
2,4-lutidine

-
-
100-47-0
benzonitrile

-
-
79-44-7
N,N-Dimethylcarbamoyl chloride

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
493-77-6
2,4,6-triphenyl-1,3,5-triazine

-
C
-
106932-30-3
methyl-4 phenyl-7 6H naphtyridin-1,6 one-5

| Conditions | Yield |
|---|---|
| With lithium 1.) diethyl ether, reflux, 1 h, 2.) ether, reflux, 2.5 h, 3.) ether; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
57-13-6
urea

-
-
1188-33-6
N,N-dimethylformamide diethyl diacetal

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
83490-19-1
N-Aminocarbonyl-N',N'-dimethyl-formamidin

-
C
-
20353-90-6
1,1-dimethyl-3-dimethylaminomethyleneurea

| Conditions | Yield |
|---|---|
| at 85℃; for 3h; Yield given. Yields of byproduct given; |

-
-
57-13-6
urea

-
-
19429-85-7
N,N-dimethylacetamide diethyl acetal

-
A
-
30885-99-5
4,6-Dimethyl-sym-triazin-2-one

-
B
-
598-94-7
1,1-Dimethylurea

-
C
-
69606-63-9
4-Dimethylamino-6-methylpyrimidin-2-one

| Conditions | Yield |
|---|---|
| for 3h; Heating; Yield given; | A n/a B n/a C 0.21 g |
| for 3h; Heating; Yields of byproduct given; | A n/a B n/a C 0.21 g |

-
-
57-13-6
urea

-
-
19429-85-7
N,N-dimethylacetamide diethyl acetal

-
A
-
30885-99-5
4,6-Dimethyl-sym-triazin-2-one

-
B
-
598-94-7
1,1-Dimethylurea

-
C
-
84690-53-9
N-Carbamoyl-N',N'-dimethylaminoacetamidine

| Conditions | Yield |
|---|---|
| In ethanol at 35 - 40℃; for 0.05h; Yield given; |

-
-
36209-52-6
3,5-dimethyl-1,3,5-oxadiazane-2,4,6-trione

-
-
598-94-7
1,1-Dimethylurea
-
-
87897-69-6
1,3-dimethyl-5-hydroxyhydantoin-5-carboxamide

-
A
-
598-94-7
1,1-Dimethylurea

-
B
-
473-90-5
acetonedicarboxylic acid

-
-
598-94-7
1,1-Dimethylurea

-
-
536-74-3
phenylacetylene

-
-
1095320-53-8
1,1-dimethyl-3-[(Z)-2-phenylvinyl]urea

| Conditions | Yield |
|---|---|
| With [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); 1,4-bis(dicyclohexylphosphino)butane; ytterbium(III) triflate In water; N,N-dimethyl-formamide at 60℃; for 6h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 99% |
| With [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); 1,4-bis(dicyclohexylphosphino)butane; water; ytterbium(III) triflate In N,N-dimethyl-formamide at 60℃; for 6h; anti-Markovnikov hydroamidation; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 46% |
-
-
598-94-7
1,1-Dimethylurea

-
-
874-60-2
4-methyl-benzoyl chloride

-
-
104825-41-4
1,1-Dimethyl-3,3-bis-(4-methyl-benzoyl)-urea

| Conditions | Yield |
|---|---|
| In pyridine for 24h; Ambient temperature; | 98% |
-
-
598-94-7
1,1-Dimethylurea

-
-
122-01-0
4-chloro-benzoyl chloride

-
-
104417-74-5
1,1-Bis-(4-chloro-benzoyl)-3,3-dimethyl-urea

| Conditions | Yield |
|---|---|
| In pyridine for 24h; Ambient temperature; | 98% |
-
-
598-94-7
1,1-Dimethylurea

-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
104825-42-5
1,1-Bis-(4-methoxy-benzoyl)-3,3-dimethyl-urea

| Conditions | Yield |
|---|---|
| In pyridine for 24h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| In chloroform | 96.7% |

-
-
13987-61-6
N-methylene-tert-butylamine

-
-
598-94-7
1,1-Dimethylurea

-
-
141564-68-3
3-{[tert-Butyl-(3,3-dimethyl-ureidomethyl)-amino]-methyl}-1,1-dimethyl-urea

| Conditions | Yield |
|---|---|
| for 4h; Heating; | 95% |
-
-
598-94-7
1,1-Dimethylurea

-
-
24303-61-5
2,2,3,3-tetramethylcyclopropanecarbonyl chloride

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Heating; | 95% |

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| In acetone stirring for 30 min at 30°C; cooling in ice; decanting; addn. of diethyl ether to soln.; drying the oily ppt. by vac. evapn.; redissolving in acetone; filtration; addn. of diethyl ether; cooling (0°C, 3 d); filtration; washing (diethyl ether); air-drying; elem. anal.; | 90% |
-
-
598-94-7
1,1-Dimethylurea

-
-
173200-35-6
1-benzyl-3,5-dichloro-6-methylpyrazin-2(1H)-one

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,2-dimethoxyethane at 80 - 100℃; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With Selectfluor; lithium iodide In dichloromethane at 80℃; for 16h; diastereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-Dimethylurea With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; Inert atmosphere; Stage #2: acridine In dimethyl sulfoxide; mineral oil at 20℃; for 24h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| In ethanol for 0.1h; Sonication; | 89% |

-
-
598-94-7
1,1-Dimethylurea

-
-
79999-47-6
tri-O-(tert-butyldimethylsilyl)-D-glucal

-
-
1391998-98-3
1,1-dimethyl-3-[3,4,6-tris(O-tert-butyldimethylsilyl)-2-deoxy-2-iodo-α-D-mannopyranosyl]urea

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In propiononitrile at -78 - 20℃; for 56h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| In ethanol for 0.1h; Sonication; | 88% |


| Conditions | Yield |
|---|---|
| In pyridine for 24h; Ambient temperature; | 86% |
-
-
598-94-7
1,1-Dimethylurea

-
-
610-14-0
2-nitrobenzyl chloride

-
-
104417-76-7
1,1-Dimethyl-3,3-bis-(2-nitro-benzoyl)-urea

| Conditions | Yield |
|---|---|
| In pyridine for 24h; Ambient temperature; | 86% |

| Conditions | Yield |
|---|---|
| In acetonitrile Ambient temperature; | 86% |

-
-
115245-01-7
trifluoromethanesulfonatopentaamminecobalt(III) trifluoromethanesulfonate

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| In water; acetone to N,N-dimethylurea in acetone (Co(NH3)5OSO2CF3)(CF3SO3)2 added and mixt. warmed (60°C) for 10 min, cooled to room temp. and poured intoEt2O, mixt. stirred for 10 min, residue redissolved in ice water and added to aq. soln. Na2S2O6; mixt. cooled (<5°C) for 15 min, crystals collected, washed with ice water, ethanol and ether and dried over P2O5; elem. anal.; | 86% |
| In acetone Co-complex added to a soln. of urea; mixture warmed at 60°C for 15 min;; poured into butan-2-ol, ether added, stirred, solid dissolved in ice-water, filtered, treated with aq. Na2S2O6 at 20°C; cooled and stirred, crystals washed with ice-water, EtOH, ether, air-dried;; | 85% |

-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; Concentration; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| In ethanol dissolving of uranium salt in a mixture of EtOH/triethylorthoformate, heating at 323 K for 90 min with stirring, addn. of urea, further heating for 90 min; cooling to room temp., filtn., addn. of ether to the filtrate with stirring, filtn., washing with ether, drying under N2, elem. anal.; | 85% |
-
-
598-94-7
1,1-Dimethylurea

-
-
2757-23-5
chloro(chlorosulfanyl)methanone

-
-
90545-89-4
5-(N,N-dimethylamino)-1,3,4-oxathiadiazol-2-one

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 20h; | 84% |
| In acetonitrile for 1h; | 74.5% |
| In acetonitrile at 20℃; | 70% |
| In methanol; hexane; dichloromethane; acetonitrile |

| Conditions | Yield |
|---|---|
| With Selectfluor; lithium iodide In dichloromethane at 80℃; for 16h; regioselective reaction; | 84% |
-
-
598-94-7
1,1-Dimethylurea

-
-
88-89-1
2,4,6-Trinitrophenol

-
-
87448-62-2
picric acid ; picrate of N,N-dimethyl-urea

| Conditions | Yield |
|---|---|
| 83% |

| Conditions | Yield |
|---|---|
| In methanol | 83% |
-
-
598-94-7
1,1-Dimethylurea

-
-
1195931-66-8
3-oxo-3H-spiro[isobenzofuran-1,9′-xanthene]-3′,6′-diyl bis(trifluoromethanesulfonate)

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 18h; Buchwald-Hartwig cross coupling; Inert atmosphere; | 83% |
-
-
598-94-7
1,1-Dimethylurea

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 5,5-dimethyl-1,3-cyclohexadiene Reflux; | 83% |
1,1-DIMETHYLUREA Chemical Properties
IUPAC Name: 1,1-Dimethylurea
The MF of 1,1-Dimethylurea (598-94-7) is C3H8N2O.
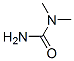
The MW of 1,1-Dimethylurea (598-94-7) is 88.11.
Synonyms of 1,1-Dimethylurea (598-94-7): 1,1-Dimethylurea ; asym-Dimethylurea ; Urea, 1,1-dimethyl-
Product Categories: Intermediates
Form: White to off-white crystalline powder
Index of Refraction: 1.452
EINECS: 202-498-7
Density: 1.023 g/ml
Flash Point: 32.7 °C
Boiling Point: 130.4 °C
Melting Point: 180-181 °C
Water Solubility: Soluble
BRN: 1740666
1,1-DIMETHYLUREA Uses
1,1-Dimethylurea (598-94-7) is used as pharmaceutical intermediates.
1,1-DIMETHYLUREA Toxicity Data With Reference
| 1. | ipr-mus LDLo:6610 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 54 (1935),188. |
1,1-DIMETHYLUREA Consensus Reports
Reported in EPA TSCA Inventory.
1,1-DIMETHYLUREA Safety Profile
Mildly toxic by intraperitoneal route. When heated to decomposition it emits toxic vapors of NOx.Safety information of 1,1-Dimethylurea (598-94-7):
Hazard Codes ![]() Xi
Xi
Risk Statements
36/37/38 Irritating to eyes, respiratory system and skin
Safety Statements
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36 Wear suitable protective clothing
WGK Germany 3
RTECS YS9867985
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 5989-54-8
- 5989-77-5
- 598-98-1
- 5989-81-1
- 59898-64-5
- 598-99-2
- 599-00-8
- 59902-16-8
- 5990-32-9
- 599-04-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View