-
Name
1,5,9-Triazacyclododecane
- EINECS
- CAS No. 294-80-4
- Article Data17
- CAS DataBase
- Density 0.852 g/cm3
- Solubility
- Melting Point 32-35 °C
- Formula C9H21N3
- Boiling Point 225.8 °C at 760 mmHg
- Molecular Weight 171.286
- Flash Point 124.9 °C
- Transport Information
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols

- Synonyms 1,5,9-Triaza-cyclododecane;
- PSA 36.09000
- LogP 0.92550
Synthetic route
-
-
127623-71-6
1,5,9-triazacyclododecane-2,4-dione

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran for 24h; Heating; | 99% |
-
-
67705-41-3
1,5,9-triazatricyclo[7.3.1.05,13 ]tridecane

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With sulfuric acid for 9.5h; Heating; | 94% |
| With hydrogenchloride for 22h; Heating; | 89% |
| With sulfuric acid In water for 5h; Reflux; | 89% |
| Stage #1: 1,5,9-triazatricyclo[7.3.1.05,13 ]tridecane With ethylene dibromide In acetonitrile Heating; Stage #2: With trifluorormethanesulfonic acid for 18h; Heating; Further stages.; |
-
-
340970-56-1
N,N',N''-tris(β-trimethylsilylethanesulfonyl)-1,5,9-triazacyclododecane

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With cesium fluoride In N,N-dimethyl-formamide at 95℃; for 24h; | 81% |
-
-
35980-67-7
1,5,9-tritosyl-1,5,9-triazacyclododecane

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With sulfuric acid at 100 - 105℃; Heating; 50-70 h; | 63% |
| With hydrogen bromide; acetic acid; phenol at 50℃; for 14h; | 63% |
| With sulfuric acid at 100℃; for 39h; | 63% |
-
-
35980-62-2
1,5,9-triazacyclododecane trihydrobromide

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol |

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With sulfuric acid at 120℃; for 30h; Yield given; |

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With ammonia; lithium Yield given; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 70 percent / sodium hydroxide / H2O / 1.) 1 hr., 5 deg C, 2.) r.t., 3 hrs. 2: 90 percent / tetrabutylammonium iodide, sodium hydroxide (50 percent) / toluene; H2O / 9 h / Heating 3: 63 percent / sulfuric acid, (conc.) / 39 h / 100 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: 90 percent / aq. NaOH / CH2Cl2 / 3 h / 20 °C 2.1: NaH / dimethylformamide / 1 h / 80 - 100 °C 2.2: 50 percent / dimethylformamide / 12 h / 100 °C 3.1: 55 percent / H2SO4 / 54 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 22 percent / sodium methoxide / ethanol / 168 h / Heating 2: 99 percent / BH3.THF / tetrahydrofuran / 24 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 63 percent / Et3N / dimethylformamide / 1.5 h / 0 °C 2: 73 percent / Cs2CO3 / dimethylformamide / 48 h / 20 °C 3: 81 percent / CsF / dimethylformamide / 24 h / 95 °C View Scheme |
-
-
35980-64-4
1,5,9-Tritosyl-1,5,9-triazanonane

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / tetrabutylammonium iodide, sodium hydroxide (50 percent) / toluene; H2O / 9 h / Heating 2: 63 percent / sulfuric acid, (conc.) / 39 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: NaH / dimethylformamide / 1 h / 80 - 100 °C 1.2: 50 percent / dimethylformamide / 12 h / 100 °C 2.1: 55 percent / H2SO4 / 54 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / Bu4NI, 50 percent aq. NaOH / toluene / 10 h / Heating 2: 63 percent / phenol, 30 percent HBr, acetic acid / 14 h / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 66 percent / 5percent NaOH / tetrabutylammonium iodide / H2O; toluene / Heating; 8-10 h 2: 63 percent / conc. H2SO4 / 100 - 105 °C / Heating; 50-70 h View Scheme |
-
-
340970-54-9
N,N',N''-tris(β-trimethylsilylethanesulfonyl)-1,5,9-triazanonane

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 73 percent / Cs2CO3 / dimethylformamide / 48 h / 20 °C 2: 81 percent / CsF / dimethylformamide / 24 h / 95 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 66 percent / KOH, KBr, NaBH4 / toluene / 42.5 h / Ambient temperature 2: 94 percent / aq. H2SO4 / 9.5 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: -20 - 20 °C 2: lithium aluminium tetrahydride / tetrahydrofuran / -78 - 20 °C 3: sulfuric acid / water / 5 h / Reflux View Scheme |

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3 / dimethylformamide / 4 h / 100 °C 2: Li, NH3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1.) HO-, 2.) H3O+ 2: BH3*S(CH3)2 3: pyridine / acetonitrile 4: KH / dimethylformamide 5: HBr 6: KOH / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: BH3*S(CH3)2 2: pyridine / acetonitrile 3: KH / dimethylformamide 4: HBr 5: KOH / methanol View Scheme |
-
-
75321-10-7
3-propyl tosylate

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: KH / dimethylformamide 2: HBr 3: KOH / methanol View Scheme |
-
-
56187-12-3
N-tosyl-bis(3-hydroxypropyl)amine

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine / acetonitrile 2: KH / dimethylformamide 3: HBr 4: KOH / methanol View Scheme |

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / -78 - 20 °C 2: sulfuric acid / water / 5 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: bis(3-aminopropyl)amine; 1,3-dibromo-propane With Phthalic acid dibutyl ester; potassium carbonate; p-toluenesulfonyl chloride Stage #2: With sulfuric acid at 90℃; for 48h; Further stages; |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |
-
-
294-80-4
1,5,9-triazacyclododecane

-
-
62051-24-5
hexahydro-3a,6a,9a-triaza-9b-phospha-phenalene

| Conditions | Yield |
|---|---|
| With Hexamethylphosphorous triamide at 120℃; for 96h; | 96% |
| With Hexamethylphosphorous triamide | |
| With Hexamethylphosphorous triamide | |
| With Hexamethylphosphorous triamide |

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In water; acetonitrile at 20℃; pH=4 - 5; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 48h; | 93% |

| Conditions | Yield |
|---|---|
| In DMF (N,N-dimethyl-formamide) at 20℃; for 48h; | A 93% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: 1,5,9-triazacyclododecane With potassium carbonate In methanol for 0.5h; Stage #2: C8H15ClN2O2 In methanol for 1h; | 92% |
-
-
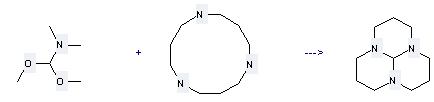
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
67705-41-3
1,5,9-triazatricyclo[7.3.1.05,13 ]tridecane

| Conditions | Yield |
|---|---|
| at 120℃; | 90% |
| at 85℃; for 3h; | 90% |
| In neat (no solvent) Heating; | 36% |
-
-
18871-66-4
N,N-dimethylacetamide dimethyl acetal

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
81115-31-3, 111264-50-7, 126188-50-9
9b-Methyl-hexahydro-3a,6a,9a-triaza-phenalene

| Conditions | Yield |
|---|---|
| Heating; | 89% |

| Conditions | Yield |
|---|---|
| In ethanol azamacrocycle in ethanol was heated and stirred at 65°C, Zn-salt in ethanol was slowly added at 65°C dropwise, stirred at 65°C for 2 h; cooled, filtered, washed with cold ethanol (3x), ppt. was dried under vac. over two days at ambient temp.; elem. anal.; | 89% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl acetamide at 80℃; | 88% |

| Conditions | Yield |
|---|---|
| In ethanol at 0℃; | 88% |
-
-
294-80-4
1,5,9-triazacyclododecane

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
97703-29-2
Mo(CO)3(NHC3H6NHC3H6NHC3H6)

| Conditions | Yield |
|---|---|
| In decalin byproducts: CO; heated to 150°C for 10 min under Ar; filtn., ppt. is washed (benzene, ether), air-dried, elem. anal.; | 85% |
-
-
17616-43-2
3-iodo-2-(iodomethyl)-1-propene

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With potassium carbonate In isopropyl alcohol Heating; | 83% |
-
-
129018-26-4
thioacetic acid S-(4-chlorocarbonyl-phenyl) ester

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
146070-03-3
1,5,9-tris(4-acetylthiobenzoyl)-1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane Ambient temperature; | 83% |

| Conditions | Yield |
|---|---|
| In water aq. soln. of ligand (0.24 mmol) and Ru compd. (0.24 mmol) added slowly to aq. soln. of Ni salt (0.24 mmol), filtered; crystd. at room temp. in darkness, filtered off, washed (methanol, Et2O), dried in air, elem. anal.; | 83% |

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
224557-85-1
O-Benzyl-2-bromo-N-methylacetohydroxamic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide 1.) 90-100 deg C, 2 h, 2.) r.t., 18 h; | 82% |
-
-
1199-01-5
2-phenylazlactone

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
174783-34-7
1,5,9-tris<(benzoylamino)acetyl>-1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| In acetonitrile for 20h; Ambient temperature; | 82% |
-
-
557-34-6
zinc diacetate

-
-
143-66-8
sodium tetraphenyl borate

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
162024-82-0
(η(3)-1,5,9-triazacyclododecane)zinc(II) acetate tetraphenylborate

| Conditions | Yield |
|---|---|
| In ethanol inert atmosphere; dropwise addn. of NaBPh4 (in THF) to mixt. of Zn-saltand ligand (in EtOH), stirring overnight; filtering, concn., addn. of Et2O (pptn.), filtering off, washing (Et2O), drying (vac.); | 80% |


-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| In hexane N2-atmosphere; addn. of equimolar amt. of dodecane derivative to Ru-complex soln. at reflux, stirring (70°C, 2 h); cooling to room temp., filtration, extn. into hexane, drying (vac.); elem. anal.; | 78.5% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; acetone for 3h; Reflux; | 78% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
174192-40-6
bis(1,1-dimethylethyl) 1,5,9-triazacyclododecane-1,5-dicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; | 72% |
| With triethylamine In chloroform for 24h; |
-
-
67217-55-4
mono-6-deoxy-6-(p-tolylsulphonyl)-β-cyclodextrin

-
-
294-80-4
1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 5h; amynation; | 72% |

| Conditions | Yield |
|---|---|
| In ethanol mixing at 50°C; recrystallized from 50% MeOH; elem. anal.; | 72% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; acetone for 3h; Reflux; | 72% |
-
-
17616-43-2
3-iodo-2-(iodomethyl)-1-propene

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
104875-18-5
11-Methylene-1,5,9-triazabicyclo[7.3.3] pentadecane

| Conditions | Yield |
|---|---|
| With potassium carbonate In isopropyl alcohol for 6h; Heating; | 70% |
| With potassium carbonate In isopropyl alcohol | 60% |
-
-
98-59-9
p-toluenesulfonyl chloride

-
-
294-80-4
1,5,9-triazacyclododecane

-
-
164913-31-9
N1,N5-ditosyl-1,5,9-triazacyclododecane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether; water at 0℃; for 1h; | 68% |
1,5,9-Triazacyclododecane Specification
The 1,5,9-Triazacyclododecane, with the CAS registry number 294-80-4, is also known as 1,5,9-Triaza-cyclododecane. It belongs to the product categories of Amine Monomers; Chelation/Complexation Compounds; Crown Ethers Monomers; Secondary Amines; Synthetic Reagents. This chemical's molecular formula is C9H21N3 and molecular weight is 171.28. What's more, its systematic name is called 1,5,9-Triazacyclododecane.
Physical properties about 1,5,9-Triazacyclododecane are: (1)ACD/LogP: 0.31; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -4.79; (4)ACD/LogD (pH 7.4): -4.66; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 3; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 9.72 Å2; (13)Index of Refraction: 1.426; (14)Molar Refractivity: 51.54 cm3; (15)Molar Volume: 200.9 cm3; (16)Surface Tension: 27.1 dyne/cm; (17)Density: 0.852 g/cm3; (18)Flash Point: 124.9 °C; (19)Enthalpy of Vaporization: 46.23 kJ/mol; (20)Boiling Point: 225.8 °C at 760 mmHg; (21)Vapour Pressure: 0.0847 mmHg at 25 °C; (22)Melting Point: 32-35 °C.
Preparation of 1,5,9-Triazacyclododecane: this chemical can be prepared by Hexahydro-3α,6α,9α-triaza-phenalene. The reaction occurs with reagent 3 M aq. HCl and other condition of heating for 22 hours. The yield is 89 %.
.jpg)
Uses of 1,5,9-Triazacyclododecane: it is used to produce other chemicals. For example, it is used to produce Hexahydro-3α,6α,9α-triaza-phenalene. This reaction needs solvent Neat and other condition of heating. The yield is 36 %.
When you are dealing with this chemical, you should be very careful. This chemical may destroy living tissue on contacting. What's more, it could cause burns. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water. And in case of other accidents or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: N1CCCNCCCNCCC1
(2) InChI: InChI=1/C9H21N3/c1-4-10-6-2-8-12-9-3-7-11-5-1/h10-12H,1-9H2
(3) InChIKey: VQFZKDXSJZVGDA-UHFFFAOYAY
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 2948-46-1
- 294857-29-7
- 294874-70-7
- 29488-24-2
- 29489-04-1
- 29489-57-4
- 29490-19-5
- 294-90-6
- 2949-11-3
- 294919-14-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View