-
Name
1-Acetamido-7-hydroxynaphthalene
- EINECS 229-293-5
- CAS No. 6470-18-4
- Article Data12
- CAS DataBase
- Density 1.297 g/cm3
- Solubility
- Melting Point 168-169 °C
- Formula C12H11NO2
- Boiling Point 486.2 °C at 760 mmHg
- Molecular Weight 201.225
- Flash Point 247.9 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetamide,N-(7-hydroxy-1-naphthyl)- (6CI,8CI);1-Acetamido-7-naphthol;1-Acetylamino-7-naphthol;N-(7-Hydroxy-1-naphthyl)acetamide;N-(7-Hydroxynaphthalene-1-yl)-acetamide;NSC 7566;acetamide, N-(7-hydroxy-1-naphthalenyl)-;
- PSA 49.33000
- LogP 2.57680
Synthetic route
-
-
29921-56-0
8-acetamidonaphthalen-2-yl acetate

-
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol for 1h; Reflux; | 96% |
| With sodium hydrogencarbonate In methanol |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 21h; | 88% |
-
-
118-46-7
8-amino-2-naphthol

-
-
75-36-5
acetyl chloride

-
A
-
29921-56-0
8-acetamidonaphthalen-2-yl acetate

-
B
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| With pyridine; benzene |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TEA / CHCl3 2: NaHCO3 (sat) / methanol View Scheme | |
| With acetic anhydride In methanol | |
| In ice-water |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / 250 - 260 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20 - 40℃; for 2h; | 120 g |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
74-88-4
methyl iodide

-
-
93189-18-5
N-(7-methoxynaphthalen-1-yl)acetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 1h; Reflux; | 90% |
| With potassium carbonate In acetone for 6h; Heating; | |
| With potassium carbonate In acetone |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 9h; Reflux; | 88% |
| With potassium carbonate In acetonitrile for 9h; Reflux; | 88% |

-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
168901-50-6
2-trifluoromethanesulfonyloxy-8-acetylaminonaphthalene

| Conditions | Yield |
|---|---|
| With dmap; trifluoromethanesulfonic acid anhydride In dichloromethane | 69.5% |
-
-
141-43-5
ethanolamine

-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
1627144-25-5
9-acetylamino-2-hydroxymethyl naphtho[2,1-d]oxazole

| Conditions | Yield |
|---|---|
| With copper dichloride In acetonitrile at 60℃; for 15h; | 62% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; 3 A molecular sieve; titanium(IV) isopropylate In dichloromethane for 16h; | 58% |
-
-
108-24-7
acetic anhydride

-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
29921-56-0
8-acetamidonaphthalen-2-yl acetate

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid |

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid |
-
-
77-78-1
dimethyl sulfate

-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
93189-18-5
N-(7-methoxynaphthalen-1-yl)acetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| bei der Behandlung des Reaktionsgemisches mit warmer Natronlauge; |
-
-
122290-34-0
diazotierte 2-Amino-benzol-disulfonsaeure-(1.4)

-
-
6470-18-4
1-acetylamino-7-naphthol
-
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| With sodium nitrite Ansaeuern mit verd. Schwefelsaeure; |
-
-
17418-58-5
C. I. disperse red 60

-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
38919-97-0
N-[7-(1-Amino-4-hydroxy-9,10-dioxo-9,10-dihydro-anthracen-2-yloxy)-naphthalen-1-yl]-acetamide

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; potassium hydroxide at 145 - 150℃; |

-
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
871732-00-2
N-(8-bromo-7-hydroxy-naphthalen-1-yl)-acetamide

| Conditions | Yield |
|---|---|
| With bromine In acetic acid for 14h; |
-
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: Br2 / acetic acid / 14 h 2.1: 4.54 g / imidazole / dimethylformamide / 1 h 3.1: MeLi / tetrahydrofuran; diethyl ether / 1 h / -78 °C 3.2: t-BuLi / tetrahydrofuran; diethyl ether; pentane / 2 h / -78 - 0 °C 3.3: 91 percent / NH4Cl / tetrahydrofuran; diethyl ether; various solvents View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
871731-86-1
N-{8-bromo-7-[[tris(1-methylethyl)silyl]oxy]}acetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Br2 / acetic acid / 14 h 2: 4.54 g / imidazole / dimethylformamide / 1 h View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
550998-29-3
3,6-dibromo-8-chloro-2-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: K2CO3 / acetone / 6 h / Heating 2: aq. HCl / 5 h / Heating 3: tert-butyl nitrite; CuCl2 / acetonitrile / 0 - 20 °C 4: 92 percent / BBr3 / CH2Cl2 / 0 - 20 °C 5: Br2; acetic acid / 1 h / 100 °C View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
550998-28-2
1,6-dibromo-8-fluoro-2-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: K2CO3 / acetone / 6 h / Heating 2.1: aq. HCl / 5 h / Heating 3.1: aq. HCl; NaNO2 / 0.5 h / 10 °C 3.2: aq. HBF4 3.3: xylene / 1 h / Heating 4.1: 3.99 g / BBr3 / CH2Cl2 / 0 - 20 °C 5.1: 62 percent / Br2; acetic acid / 1 h / 100 °C View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: K2CO3 / acetone / 6 h / Heating 2: aq. HCl / 5 h / Heating 3: tert-butyl nitrite; CuCl2 / acetonitrile / 0 - 20 °C 4: 92 percent / BBr3 / CH2Cl2 / 0 - 20 °C 5: 1.80 g / Br2; acetic acid / 1 h / 100 °C View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
550998-42-0
8-chloro-6-(4-methoxyphenyl)-2-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: K2CO3 / acetone / 6 h / Heating 2.1: aq. HCl / 5 h / Heating 3.1: tert-butyl nitrite; CuCl2 / acetonitrile / 0 - 20 °C 4.1: 92 percent / BBr3 / CH2Cl2 / 0 - 20 °C 5.1: Br2; acetic acid / 1 h / 100 °C 6.1: 84 percent / imidazole / dimethylformamide / 3 h 7.1: 69 percent / Pd[P(Ph)3]4 / tetrahydrofuran / 3 h / Heating 8.1: 88 percent / TBAF / tetrahydrofuran / 0.17 h / 20 °C 9.1: t-BuLi / tetrahydrofuran / 0.33 h / -78 °C 9.2: H2O / tetrahydrofuran / -78 - 20 °C View Scheme |
-
-
6470-18-4
1-acetylamino-7-naphthol

-
-
550998-32-8
8-fluoro-6-(4-methoxyphenyl)-2-naphthol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: K2CO3 / acetone / 6 h / Heating 2.1: aq. HCl / 5 h / Heating 3.1: aq. HCl; NaNO2 / 0.5 h / 10 °C 3.2: aq. HBF4 3.3: xylene / 1 h / Heating 4.1: 3.99 g / BBr3 / CH2Cl2 / 0 - 20 °C 5.1: 62 percent / Br2; acetic acid / 1 h / 100 °C 6.1: 41 percent / SnCl2; aq. HCl; acetic acid / 1 h / 100 °C 7.1: 81 percent / aq. Na2CO3; Pd[P(Ph)3]4 / 1,2-dimethoxy-ethane / 85 °C View Scheme |
1-Acetamido-7-hydroxynaphthalene Standards and Recommendations
Content: 95% min
Purity: 97% min
Alkali insoluble matters: 0.1% max
1-Acetamido-7-hydroxynaphthalene Specification
The 1-Acetamido-7-hydroxynaphthalene, with the CAS registry number 6470-18-4 and EINECS registry number 229-293-5, has the systematic name of N-(7-hydroxynaphthalen-1-yl)acetamide. And the molecular formula of this chemical is C12H11NO2. It belongs to the product categories Intermediates of Dyes and Pigments. What's more, it should be used as intermediate of coupling component in neutral stain.
The physical properties of 1-Acetamido-7-hydroxynaphthalene are as following: (1)ACD/LogP: 1.58; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.58; (4)ACD/LogD (pH 7.4): 1.57; (5)ACD/BCF (pH 5.5): 9.3; (6)ACD/BCF (pH 7.4): 9.23; (7)ACD/KOC (pH 5.5): 171.7; (8)ACD/KOC (pH 7.4): 170.41; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 29.54 Å2; (13)Index of Refraction: 1.704; (14)Molar Refractivity: 60.24 cm3; (15)Molar Volume: 155 cm3; (16)Polarizability: 23.88×10-24cm3; (17)Surface Tension: 59 dyne/cm; (18)Density: 1.297 g/cm3; (19)Flash Point: 247.9 °C; (20)Enthalpy of Vaporization: 78.05 kJ/mol; (21)Boiling Point: 486.2 °C at 760 mmHg; (22)Vapour Pressure: 4.45E-10 mmHg at 25°C.
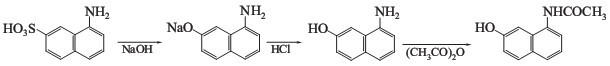
Preparation of 1-Acetamido-7-hydroxynaphthalene: It can start with alkaline fusion of 1,7-cleve's acid, and gives the 8-amino-2-naphthol sodium salt. Then acidatewith dilute acid, and the 8-amino-2-naphthol will separate out. Acetylize it with acetic anhydride, and gives the desired product.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(Nc1c2c(ccc1)ccc(O)c2)C
(2)InChI: InChI=1/C12H11NO2/c1-8(14)13-12-4-2-3-9-5-6-10(15)7-11(9)12/h2-7,15H,1H3,(H,13,14)
(3)InChIKey: ALNWQAFPXMGLTJ-UHFFFAOYAR
Related Products
- 1-Acetamido-7-hydroxynaphthalene
- 6470-20-8
- 647032-09-5
- 64704-12-7
- 64704-23-0
- 64704-41-2
- 6470-52-6
- 6470-53-7
- 64706-54-3
- 6470-90-2
- 64709-57-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View