-
Name
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide
- EINECS
- CAS No. 3688-53-7
- Density 1.453g/cm3
-
Solubility
Stability
- Stable. Hydrophobic.
Toxicology
- Harmful if swallowed or inhaled. Experimental carcinogenand neoplastigen. Possible human carcinogen.
- Melting Point 151-152oC
- Formula C11H8 N2 O5
- Boiling Point 459.6°Cat760mmHg
- Molecular Weight 248.195
- Flash Point 231.8°C
- Transport Information
- Appearance orange crystalline solid
- Safety Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and teratogenic data. Poison by ingestion. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
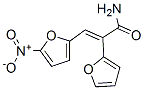
Molecular Structure
- Hazard Symbols
- Synonyms 2-Furanacrylamide,a-2-furyl-5-nitro- (7CI,8CI);2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide; 2-(2-Furyl)-3-(5-nitrofuryl)acrylamide;AF 2; AF 2 (preservative); Furylamide; Furylfuramide; NSC 44973
- PSA 115.19000
- LogP 3.03020
Toxicity data
(Th
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Chemical Properties
IUPAC Name: 2-(Furan-2-yl)-3-(5-nitrofuran-2-yl)prop-2-enamide
The MF of 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (CAS NO.3688-53-7) is C11H8N2O5.
The MW of 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (CAS NO.3688-53-7) is 248.19.
Synonyms of 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (CAS NO.3688-53-7): Furylfuramide ; 2-Furanacetamide, alpha-((5-nitro-2-furanyl)methylene)- ; cis-3-(5-Nitro-2-furyl)-2-(2-furyl)acrylamide ; alpha-((5-nitro-2-furanyl)methylene)-2-furanacetamide
Apperance: Bright orange crystals
Stability: Stable. Hydrophobic
Index of Refraction: 1.641
Density: 1.453 g/ml
Flash Point: 231.8 °C
Boiling Point: 459.6 °C
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Uses
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (CAS NO.3688-53-7) is used as fine chemical raw materials, pharmaceutical intermediates, organic synthesis.
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Toxicity Data With Reference
| 1. | mmo-sat 4 µg/L | MUREAV Mutation Research. 147 (1985),219. | ||
| 2. | sce-hmn:lym 500 µg/L | CNREA8 Cancer Research. 40 (1980),4775. | ||
| 3. | scu-mus TDLo:150 mg/kg (13-17D preg):NEO,TER | NATUAS Nature. 258 (1975),610. | ||
| 4. | orl-rat LD50:1554 mg/kg | TJEMAO Tohoku Journal of Experimental Medicine. 103 (1971),331. | ||
| 5. | orl-mus LD50:221 mg/kg | NEZAAQ Nippon Eiseigaku Zasshi. Japanese Journal of Hygiene. 28 (1973),463. |
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Consensus Reports
IARC Cancer Review: Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 31 (1983),p. 47.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 31 (1983),p. 47.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program.
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and teratogenic data. Poison by ingestion. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide Specification
A nitrated amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Related Products
- 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide
- 368866-07-3
- 368866-17-5
- 368866-32-4
- 368866-35-7
- 36886-76-7
- 368869-85-6
- 368869-86-7
- 368869-87-8
- 368869-88-9
- 368869-89-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View