-
Name
2,3,4-Trifluorobenzenamine
- EINECS 253-703-1
- CAS No. 3862-73-5
- Article Data15
- CAS DataBase
- Density 1.409 g/cm3
- Solubility
- Melting Point 14-15°C
- Formula C6H4F3N
- Boiling Point 173.8 °C at 760 mmHg
- Molecular Weight 147.1
- Flash Point 71.8 °C
- Transport Information UN 3082 9/PG 3
- Appearance Pale yellow liquid
- Safety 23-26-36/37/39-61
- Risk Codes 21/22-38-41-48/22-51/53
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  Xi;
Xi;  N
N
- Synonyms 4-12-00-01114 (Beilstein Handbook Reference);Aniline, 2,3,4-trifluoro-;Benzenamine, 2,3,4-trifluoro-;2,3,4-TFA;
- PSA 26.02000
- LogP 2.26730
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hypophosphite monohydrate; 1% platinum on charcoal; hydrogen at 65 - 80℃; under 6750.68 Torr; for 2.75h; Reagent/catalyst; Temperature; Autoclave; | 98.52% |
| With hydrogen; nickel Autoclave; | 91.8% |
| With iron; ammonium chloride |
-
-
367-25-9
2,4-difluorophenylamine

-
A
-
367-34-0
2,4,5-trifluoroaniline

-
B
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; fluorine at 20℃; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: F2; TfOH / 20 °C 2: F2; TfOH / 20 °C 3: F2; TfOH / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: F2; TfOH / 20 °C 2: F2; TfOH / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid; aqueous nitric acid / 40 °C 2: dimethylformamide; potassium fluoride / 150 °C 3: iron; aqueous NH4Cl View Scheme | |
| Multi-step reaction with 3 steps 1: sulfuric acid; nitric acid / 2 h / Heating 2: sulfolane; potassium fluoride / 8 h / 200 - 210 °C 3: nickel; hydrogen / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide; potassium fluoride / 150 °C 2: iron; aqueous NH4Cl View Scheme | |
| Multi-step reaction with 2 steps 1: sulfolane; potassium fluoride / 8 h / 200 - 210 °C 2: nickel; hydrogen / Autoclave View Scheme |
-
-
771-69-7
2,3,4-trifluoronitrobenzene

-
A
-
369-34-6
3,4-difluoronitrobenzene

-
B
-
6921-22-8
1,2-difluoro-3-nitrobenzene

-
C
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| With Dimethylphenylsilane; o-phenylenebis(diphenylphosphine); potassium tert-butylate; copper(l) chloride In tetrahydrofuran for 12h; Inert atmosphere; Reflux; Overall yield = 95 %Spectr.; regioselective reaction; |

-
-
20098-48-0
3,4,5-trichloronitrobenzen

-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: potassium fluoride; N-benzyl-N,N,N-triethylammonium chloride / 11 h / 140 - 175 °C 2.1: hydrogen / methanol / Inert atmosphere 3.1: nitrosylsulfuric acid / 6 h / 10 - 15 °C 3.2: 1 h 4.1: sulfuric acid; nitric acid / 2 h / Heating 5.1: sulfolane; potassium fluoride / 8 h / 200 - 210 °C 6.1: nickel; hydrogen / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogen / methanol / Inert atmosphere 2.1: nitrosylsulfuric acid / 6 h / 10 - 15 °C 2.2: 1 h 3.1: sulfuric acid; nitric acid / 2 h / Heating 4.1: sulfolane; potassium fluoride / 8 h / 200 - 210 °C 5.1: nickel; hydrogen / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: nitrosylsulfuric acid / 6 h / 10 - 15 °C 1.2: 1 h 2.1: sulfuric acid; nitric acid / 2 h / Heating 3.1: sulfolane; potassium fluoride / 8 h / 200 - 210 °C 4.1: nickel; hydrogen / Autoclave View Scheme |
-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
108-24-7
acetic anhydride

-
-
365-29-7
N-(2,3,4-trifluorophenyl)acetamide

| Conditions | Yield |
|---|---|
| In chloroform | 99% |
| With dmap at 0 - 20℃; for 6.16667h; | 95% |
| at 20℃; for 0.25h; | 58% |
| With hydrogenchloride at 90℃; |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 120℃; for 0.133333h; microwave irradiation; | 99% |
| In N,N-dimethyl-formamide at 120℃; for 4h; |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 18h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With 2-((dicyclohexylphosphino)methyl)-1,3-bis(2,6-diisopropylphenyl)-4,5-dimethyl-1H-imidazol-3-ium iodide; palladium diacetate; sodium t-butanolate In 1,4-dioxane at 120℃; for 20h; Buchwald-Hartwig amination; Inert atmosphere; chemoselective reaction; | 95% |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-trifluoroaniline With magnesium nitrite In water at 30℃; Acidic conditions; Flow reactor; Stage #2: With sodium sulfite In water at 90 - 110℃; Stage #3: With hydrogenchloride In water at 120℃; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0℃; for 1h; | 95% |
| With sodium hydrogencarbonate In water; ethyl acetate at 20℃; for 1h; |
-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
140-89-6
potassium ethyl xanthogenate

-
-
85118-01-0
alpha-bromo-3,4-difluorotoluene

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-trifluoroaniline; potassium ethyl xanthogenate In N,N-dimethyl-formamide at 120℃; for 0.133333h; microwave irradiation; Stage #2: alpha-bromo-3,4-difluorotoluene In N,N-dimethyl-formamide at 90℃; for 0.1h; microwave irradiation; | 93% |

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
530102-75-1
C6H2F3NOS

| Conditions | Yield |
|---|---|
| With thionyl chloride In benzene Michaelis reaction; | 92% |
-
-
446-48-0
o-fluorobenzyl bromide

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
140-89-6
potassium ethyl xanthogenate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-trifluoroaniline; potassium ethyl xanthogenate In N,N-dimethyl-formamide at 120℃; for 0.133333h; microwave irradiation; Stage #2: o-fluorobenzyl bromide In N,N-dimethyl-formamide at 90℃; for 0.1h; microwave irradiation; | 92% |

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
119474-40-7
2,3,4-trifluorophenyl isothiocyanate

-
-
354151-69-2
N,N'-Di(2,3,4-trifluorophenyl)thiourea

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 91% |
| In ethanol at 20℃; for 8h; |
-
-
610-97-9
o-iodo-methyl-benzoic acid

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
741281-01-6
methyl 2-(2,3,4-trifluoroanilino)benzoate

| Conditions | Yield |
|---|---|
| With DPE-Phos; caesium carbonate; palladium diacetate In toluene at 95℃; for 48h; Buchwald-Hartwig amination reaction; | 91% |
-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
58756-32-4
1,2,3-thiadiazole-5-carbonyl azide

| Conditions | Yield |
|---|---|
| In toluene Heating; | 91% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; sulfuric acid In tetrahydrofuran for 1.66667h; Ambient temperature; | 90% |
-
-
85118-00-9
2,6-Difluorobenzyl bromide

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
140-89-6
potassium ethyl xanthogenate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,4-trifluoroaniline; potassium ethyl xanthogenate In N,N-dimethyl-formamide at 120℃; for 0.133333h; microwave irradiation; Stage #2: 2,6-Difluorobenzyl bromide In N,N-dimethyl-formamide at 90℃; for 0.1h; microwave irradiation; | 90% |

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
372-09-8
cyanoacetic acid

-
-
239081-10-8
2-cyano-N-[(2,3,4-trifluoro)phenyl]acetamide

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid With phosphorus pentachloride In dichloromethane for 0.5h; Reflux; Stage #2: 2,3,4-trifluoroaniline In dichloromethane for 2h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 72h; Molecular sieve; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With sodium borodeuteride; sulfuric acid In tetrahydrofuran for 1.66667h; Ambient temperature; | 89% |
-
-
75-15-0
carbon disulfide

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
121-44-8
triethylamine

-
-
119474-39-4
triethylammonium N-(2,3,4-trifluorophenyl)dithiocarbamate

| Conditions | Yield |
|---|---|
| for 144h; Ambient temperature; | 89% |
| for 80h; Ambient temperature; | 87% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 1h; Heating; | 89% |
-
-
37942-07-7
3,5-di-tert-butyl-2-hydroxybenzaldehyde

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
1229248-02-5
N-2,3,4-trifluorophenyl-3,5-di-tert-butylsalicylaldimine

| Conditions | Yield |
|---|---|
| With formic acid In ethanol for 36h; Reflux; | 88% |
| With formic acid In ethanol Reflux; |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| With iodine; iodic acid In 1,4-dioxane; water for 5h; Reflux; | 88% |
| With N-iodo-succinimide In N,N-dimethyl-formamide at 20℃; for 3h; | 38% |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| at 20℃; | 87% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 20℃; for 8h; Cooling with ice; | 86.8% |
-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
100501-60-8
diethyl 2-((2,3,4-trifluorophenylamino)methylene)malonate

| Conditions | Yield |
|---|---|
| for 2h; Heating; | 86% |
| at 120 - 130℃; for 3h; | 80% |
| at 110 - 120℃; for 2h; | 74% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 7h; Reflux; | 86% |

| Conditions | Yield |
|---|---|
| In toluene at 60℃; for 4h; | 85.8% |
-
-
14220-64-5, 39958-10-6, 15617-18-2
bis(benzonitrile)palladium(II) chloride

-
-
3862-73-5
2,3,4-trifluoroaniline

-
-
919990-61-7
trans-[PdCl2(2,3,4-trifluoroaniline)2]

| Conditions | Yield |
|---|---|
| In ethanol under N2; soln. of PdCl2(C6H5CN)2 (0.26 mmol) added to soln. of 2,3,5-trifluoroaniline (0.52 mmol); refluxed (8 h); hot soln. filtered; solvent removed (vac.); elem. anal.; | 85% |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| In toluene for 4h; Reflux; | 84.2% |
-
-
3862-73-5
2,3,4-trifluoroaniline

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 5℃; for 2h; | 83.11% |
2,3,4-Trifluoroaniline Specification
The IUPAC name of this chemical is 2,3,4-Trifluoroaniline. With the CAS registry number 3862-73-5, it is also named as Aniline, 2,3,4-trifluoro-. In addition, the molecular formula is C6H4F3N and the molecular weight is 147.10. It is a kind of clear purple to brown liquid and belongs to the classes of Anilines, Aromatic Amines and Nitro Compounds; Fluorobenzene; Amines; C2 to C6; Nitrogen Compounds. And it should be stored in a cool, ventilated and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 1.79; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.79; (4)ACD/LogD (pH 7.4): 1.79; (5)ACD/BCF (pH 5.5): 13.59; (6)ACD/BCF (pH 7.4): 13.6; (7)ACD/KOC (pH 5.5): 225.31; (8)ACD/KOC (pH 7.4): 225.43; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.495; (14)Molar Refractivity: 30.47 cm3; (15)Molar Volume: 104.3 cm3; (16)Polarizability: 12.07 ×10-24cm3; (17)Surface Tension: 35.3 dyne/cm; (18)Density: 1.409 g/cm3; (19)Flash Point: 71.8 °C; (20)Enthalpy of Vaporization: 41.01 kJ/mol; (21)Boiling Point: 173.8 °C at 760 mmHg; (22)Vapour Pressure: 1.25 mmHg at 25°C.
Preparation of 2,3,4-Trifluoroaniline: it can be prepared by 1,2,3-trifluorine-4-nitrobenzene through reduction.
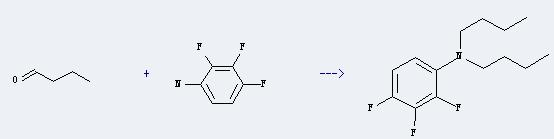
Uses of 2,3,4-Trifluoroaniline: it can be used as intermediate of norfloxacin. And it can to used to synthetize ofloxacin which is a kind of antimicrobial. In addition, it can react with butyraldehyde to get dibutyl-(2,3,4-trifluoro-phenyl)-amine. This reaction will need reagents 3 M H2SO4 and NaBH4 and solvent tetrahydrofuran. The reaction time is 100 minutes at ambient temperature. The yield is about 90%.
When you are using this chemical, please be cautious about it as the following:
It is harmful in contact with skin and if swallowed, irritating to skin and toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. And it has risk of serious damage to the eyes and danger of serious damage to health by prolonged exposure if swallowed. During using it, do not breathe vapour and wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition,avoid release to the environment and refer to special instructions/safety data sheets.
You can still convert the following datas into molecular structure:
(1)SMILES: Fc1c(F)ccc(N)c1F
(2)InChI: InChI=1/C6H4F3N/c7-3-1-2-4(10)6(9)5(3)8/h1-2H,10H2
(3)InChIKey: WRDGNXCXTDDYBZ-UHFFFAOYAZ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 699mg/kg (699mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: CYANOSIS GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 61, 1990. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 38628-51-2
- 3863-11-4
- 38632-00-7
- 38635-54-0
- 38636-12-3
- 3863-80-7
- 38641-16-6
- 38641-82-6
- 38641-94-0
- 38642-49-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View