-
Name
4-Aminopyrazolo[3,4-d]pyrimidine
- EINECS 219-174-6
- CAS No. 2380-63-4
- Article Data47
- CAS DataBase
- Density 1.612 g/cm3
- Solubility Insoluble in water.
- Melting Point >325 °C(lit.)
- Formula C5H5N5
- Boiling Point 431.088 °C at 760 mmHg
- Molecular Weight 135.128
- Flash Point 244.382 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance Light tan solid
- Safety 26-36/37/39-45-22
- Risk Codes 25-36/37/38-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 1H-Pyrazolo[3,4-d]pyrimidine,4-amino- (6CI,7CI,8CI);1H-Pyrazolo[3,4-d]pyrimidin-4-ylamine;8-Aza-7-deazaadenine;NSC 1393;1H-Pyrazolo[3,4-d]pyrimidin-4-amine;
- PSA 80.48000
- LogP 0.51630
Synthetic route
-
-
16617-46-2
3-Amino-4-cyanopyrazole

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| at 180℃; | 100% |
| at 170℃; for 5h; Inert atmosphere; | 94% |
| for 1h; Reflux; | 93% |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
16617-46-2
3-amino-4-cyano-2H-pyrazole

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| at 180℃; Inert atmosphere; | 95% |
| at 180℃; for 2h; Microwave irradiation; | 93% |
| at 180℃; for 2h; Microwave irradiation; | 93% |
-
-
16617-46-2
3-Amino-4-cyanopyrazole

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| In formamide | 87% |
-
-
16617-46-2
3-Amino-4-cyanopyrazole

-
B
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| In formamide | A n/a B 87% |
-
-
3473-63-0
formamidine acetic acid

-
-
16617-46-2
3-amino-4-cyano-2H-pyrazole

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| at 120℃; for 45h; Temperature; Concentration; Inert atmosphere; | 84.1% |
| In ethanol for 5h; Reflux; | 75% |
-
-
78972-81-3
N1,N1-dimethyl-N2-[4-cyanopyrazol-5-yl]formamidine

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| With ammonium hydroxide for 5h; Heating; | 80% |
-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
83255-86-1
3-bromo-4-aminopyrazolo<3,4-d>pyrimidine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 60℃; for 3h; | 100% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 80℃; for 5h; | 92% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 60℃; | 91% |
-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
151266-23-8
4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidine

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In N,N-dimethyl-formamide at 80℃; | 100% |
| With N-iodo-succinimide In N,N-dimethyl-formamide at 80℃; for 5h; | 98% |
| With N-iodo-succinimide In N,N-dimethyl-formamide for 12h; | 97% |
-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
684-16-2
Hexafluoroacetone

-
-
125509-32-2
2,2,4,4-tetrakis(trifluoromethyl)-2,8-dihydro<1,3,5>oxadiazino<3,4-c>pyrazolopyrimidine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 20℃; Inert atmosphere; | 90% |
-
-
1373333-65-3
[(3aR,4S,6R, 6aR)-2,2-dimethyl-6-vinyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl] trifluoromethanesulfonate

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminopyrazolo<3,4-d>pyrimidin With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Mitsunobu Displacement; Stage #2: [(3aR,4S,6R, 6aR)-2,2-dimethyl-6-vinyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl] trifluoromethanesulfonate In dichloromethane; N,N-dimethyl-formamide; mineral oil at 20℃; for 16h; Mitsunobu Displacement; | 88% |
-
-
516-12-1
N-iodo-succinimide

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
151266-23-8
4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; for 12h; | 86% |
| In N,N-dimethyl-formamide at 80℃; Concentration; Solvent; |
-
-
2974-94-9
4-phenoxyiodobenzene

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
330786-24-8
3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 120℃; for 24h; Inert atmosphere; | 82% |
-
-
137-43-9
Cyclopentyl bromide

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
21254-14-8
1-cyclopentyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 110℃; | 80% |
-
-
6974-32-9
1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
854020-39-6
(2R,3R,4R,5R)-2-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-1-yl)-5-((benzoyloxy)methyl)tetrahydrofuran-3,4-diyl dibenzoate

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In nitromethane for 2h; Heating; | 79% |
| With boron trifluoride diethyl etherate In nitromethane for 1.5h; Reflux; | 59% |
| With boron trifluoride diethyl etherate In nitromethane Reflux; | 42% |

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminopyrazolo<3,4-d>pyrimidin With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Stage #2: C16H18F4O5S In N,N-dimethyl-formamide; mineral oil at 20℃; for 2h; | 78% |
-
-
961-07-9
2'-Deoxyguanosine

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
17318-21-7
4-amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1H-pyrazolo[3,4-d]pyrimidine

| Conditions | Yield |
|---|---|
| With purine nucleoside phosphorylase at 50℃; pH=7; aq. phosphate buffer; Enzymatic reaction; | 77% |

-
-
166826-77-3
(carboxymethyl(4-methylbenzyl)amino)acetic acid

-
-
7732-18-5
water

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| Stage #1: copper (II) carbonate hydroxide; (carboxymethyl(4-methylbenzyl)amino)acetic acid In water at 50℃; Stage #2: water; 4-Aminopyrazolo<3,4-d>pyrimidin In isopropyl alcohol for 1h; | 75% |

-
-
129888-60-4
1-(tert-Butoxycarbonyl)-3-(methanesulfonyloxy)piperidine

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
1374250-98-2
tert-butyl 3-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; | 72% |
| Stage #1: 4-Aminopyrazolo<3,4-d>pyrimidin With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Stage #2: 1-(tert-Butoxycarbonyl)-3-(methanesulfonyloxy)piperidine In N,N-dimethyl-formamide; mineral oil at 100℃; for 16h; | 50% |
-
-
96-49-1
[1,3]-dioxolan-2-one

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
100524-24-1
9-(2'-hydroxyethyl)adenine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide for 2h; Heating; | 71% |

-
-
7732-18-5
water

-
-
1813-23-6
(carboxymethyl(4-fluorobenzyl)amino)acetic acid

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| Stage #1: copper (II) carbonate hydroxide; (carboxymethyl(4-fluorobenzyl)amino)acetic acid In water at 50℃; Stage #2: water; 4-Aminopyrazolo<3,4-d>pyrimidin In isopropyl alcohol for 1h; | 65% |

-
-
80044-09-3
1-azido-2,3-epoxypropane

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 8h; | 64% |
-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
98-80-6
phenylboronic acid

-
-
99973-41-8
N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; copper diacetate; caesium carbonate In N,N-dimethyl-formamide at 60℃; for 12h; Chan-Lam Coupling; | 62% |
-
-
109-01-3
1-methyl-piperazine

-
-
50-00-0
formaldehyd

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
83255-90-7
1-(N-methylpiperazinomethyl)-4-(N-methylpiperazinomethylamino)pyrazolo<3,4-d>pyrimidine

| Conditions | Yield |
|---|---|
| In water | 60% |

-
-
951-78-0
2'-deoxyuridine

-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
17318-21-7
4-amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1H-pyrazolo[3,4-d]pyrimidine

| Conditions | Yield |
|---|---|
| With purine nucleoside phosphorylase In dimethyl sulfoxide at 50℃; for 72h; pH=7; aq. phosphate buffer; Enzymatic reaction; | 60% |
| at 37℃; for 16h; alginate gel-entrapped cells of auxotrophic thymine-dependent strain of E. coli, ammonium acetate buffer pH 5.7; |
-
-
2380-63-4
4-Aminopyrazolo<3,4-d>pyrimidin

-
-
100-51-6
benzyl alcohol

-
-
58360-86-4
N-benzyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With samarium diiodide; potassium tert-butylate In tetrahydrofuran; toluene at 140℃; for 1.5h; Microwave irradiation; Inert atmosphere; | 59% |
4-Aminopyrazolo[3,4-d]pyrimidine Chemical Properties
IUPAC Name:1H-Pyrazolo[3,4-d]pyrimidin-4-amine
Synonyms: 4-Aminopyrazolo(3,4-d)pyrimidine ; 1H-Pyrazolo(3,4-d)pyrimidin-4-amine ; 1H-Pyrazolo(3,4-d)pyrimidine, 4-amino- ;1H-pyrazolo[3,4-d]pyrimidin-4-amine ; 1H-Pyrazolo[3,4-d]pyrimidin-4-ylamine ; 1H-Pyrazolo[3,4-d]pyrimidine, 4-amino-
Product Categories: Pyrimidine ;Pyridines, Pyrimidines, Purines and Pteredines;Heterocyclic Compounds;Heterocycles
Mol File: 2380-63-4.mol
Molecular Structure : 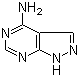
Molecular Formula: C5H5N5
Molecular Weight: 135.13
CAS NO: 2380-63-4
EINECS : 219-174-6
BRN : 5824
Mol File: 2380-63-4.mol
Index of Refraction: 1.837
Surface Tension: 122.7 dyne/cm
Density: 1.612 g/cm3
Flash Point: 244.4 °C
Enthalpy of Vaporization: 68.67 kJ/mol
Boiling Point: 431.1 °C at 760 mmHg
Vapour Pressure: 1.23E-07 mmHg at 25°C
Melting point: >325 °C(lit.)
Appearance: Pyrazoloadenine (CAS NO. 2380-63-4) is light tan solid.
4-Aminopyrazolo[3,4-d]pyrimidine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 100mg/kg (100mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 398, 1956. |
| mouse | LD10 | unreported | 15mg/kg (15mg/kg) | LIVER: OTHER CHANGES | Progress in Medical Chemistry. Vol. 7, Pg. 69, 1970. |
| mouse | LD50 | intraperitoneal | 181mg/kg (181mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | oral | 180mg/kg (180mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CILIARY SPASM: EYE SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: EXCITEMENT | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 398, 1956. |
| rat | LD50 | intraperitoneal | 228mg/kg (228mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CILIARY SPASM: EYE SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: EXCITEMENT | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 398, 1956. |
| rat | LD50 | oral | 141mg/kg (141mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CILIARY SPASM: EYE SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: EXCITEMENT | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 398, 1956. |
4-Aminopyrazolo[3,4-d]pyrimidine Consensus Reports
Reported in EPA TSCA Inventory.
4-Aminopyrazolo[3,4-d]pyrimidine Safety Profile
Poison by ingestion and intraperitoneal routes. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes  T
T
Risk Statements 36/37/38-23/24/25
R36/37/38: Pyrazoloadenine (CAS NO. 2380-63-4) is irritating to eyes, respiratory system and skin.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
Safety Statements 26-36/37/39-45-22
S22:Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
RIDADR UN 2811 6.1/PG 3
WGK Germany 3
RTECS UR0717000
HazardClass 6.1
PackingGroup III
4-Aminopyrazolo[3,4-d]pyrimidine Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use carbon dioxide or dry chemical.
Handling: Avoid contact with skin and eyes. Do not breathe dust, vapor, mist, or gas.
Storage: Store Pyrazoloadenine (CAS NO. 2380-63-4) in a cool, dry place. Store in a tightly closed container.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View