-
Name
4-Chloro-2-nitrobenzaldehyde
- EINECS 226-915-7
- CAS No. 5551-11-1
- Article Data38
- CAS DataBase
- Density 1.485 g/cm3
- Solubility
- Melting Point 68 °C
- Formula C7H4ClNO3
- Boiling Point 305.2 °C at 760 mmHg
- Molecular Weight 185.567
- Flash Point 138.4 °C
- Transport Information
- Appearance white crystalline powder
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Nitro-4-chlorobenzaldehyde;
- PSA 62.89000
- LogP 2.58390
Synthetic route
-
-
22996-18-5
(4-chloro-2-nitrophenyl)methanol

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 4,4'-Dimethoxy-2,2'-bipyridin; 9-azabicyclo[3.3.1]nonane N-oxyl; oxygen In acetonitrile at 50℃; for 2.2h; | 96% |
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 9-azabicyclo<3.3.1>nonane-N-oxyl In acetonitrile at 50℃; for 4h; Sealed tube; | 95% |
| With manganese(IV) oxide | 66% |
| With pyridinium chlorochromate In dichloromethane for 2h; Ambient temperature; | 57% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chloro-2-nitrotoluene With N,N-dimethyl-aniline In N,N-dimethyl-formamide at 140℃; for 48h; Stage #2: With sodium periodate In water; N,N-dimethyl-formamide at 20℃; for 4h; | 90% |
| Stage #1: 4-chloro-2-nitrotoluene With N,N-dimethyl-formamide dimethyl acetal In N,N-dimethyl-formamide at 140℃; for 16h; Stage #2: With sodium periodate In N,N-dimethyl-formamide at 0 - 20℃; Further stages.; | 84% |
| With N,N-dimethyl-formamide dimethyl acetal In N,N-dimethyl-formamide at 135℃; for 48h; Temperature; | 54% |
-
-
89-59-8
4-chloro-2-nitrotoluene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4-chloro-2-nitrotoluene; N,N-dimethyl-formamide dimethyl acetal In N,N-dimethyl-formamide at 140℃; for 16h; Stage #2: With sodium periodate In tetrahydrofuran; water at 0 - 20℃; for 5.16667h; | 84% |
-
-
52311-59-8
4-chloro-2-nitrobenzyl bromide

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With hexamethylenetetramine In acetic acid for 4h; Heating; | 40% |
-
-
807639-75-4
4-chloro-2-nitro-benzaldehyde-oxime

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With iron (III)-ammonium sulfate; sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
959089-98-6
4-amino-2-nitro-benzaldehyde-oxime

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iron(III) chloride | |
| Diazotization.Verkochen der Diazoniumverbindung in Saeure mit Cuprochlorid; |
-
-
116435-81-5
(4-amino-2-nitrophenyl)acetic acid

-
A
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
B
-
37777-71-2
4-chloro-2-nitro-phenylacetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; anschliessendes Erhitzen mit Wasserdampf; | |
| With hydrogenchloride; copper(l) chloride Erhitzen des Reaktionsgemisches mit Wasserdampf; |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid; water |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| (i) aq. NaOH, N,N-dimethyl-4-nitroso-aniline, (ii) aq. HCl; Multistep reaction; |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde
-
-
7647-01-0
hydrogenchloride

-
-
959089-98-6
4-amino-2-nitro-benzaldehyde-oxime

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
7647-01-0
hydrogenchloride

-
-
116435-81-5
(4-amino-2-nitrophenyl)acetic acid

-
A
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
B
-
37777-71-2
4-chloro-2-nitro-phenylacetic acid

| Conditions | Yield |
|---|---|
| anschliessend Behandeln mit Kupfer(I)-chlorid und Erhitzen des Reaktionsgemisches mit Wasserdampf; |

-
-
32989-56-3
5-chloro-trans-2-[β-(dimethylamino)vinyl]-nitrobenzene

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium periodate In water; N,N-dimethyl-formamide at 20℃; for 3h; | 22.4 g |
| With sodium periodate In tetrahydrofuran; water at 20℃; for 2h; |
-
-
32989-56-3
4-chloro-1-[(2-dimethylamino)ethenyl]-2-nitrobenzene

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium periodate In water; N,N-dimethyl-formamide at 20℃; for 3h; | 2.68 g |
| With sodium periodate In water; N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; | 2.15 g |
| With dihydrogen peroxide In acetonitrile at 25℃; for 5h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 11 h / 135 °C 2: 22.4 g / NaIO4 / H2O; dimethylformamide / 3 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 45 percent / NBS, benzoyl peroxide / CCl4 / Heating 2: 40 percent / HMTA / aq. acetic acid / 4 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous ammonium polysulfide solution 2: aqueous hydrochloric acid; aqueous copper (I)-chloride-solution / Erhitzen des Reaktionsgemisches mit Wasserdampf View Scheme |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
150869-41-3
methyl (E)-3-(4-chloro-2-nitrophenyl)acrylate

| Conditions | Yield |
|---|---|
| In toluene for 1h; Heating; | 98% |
| In toluene Heating; | 82% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
36646-78-3
3-(p-tolylamino)-5,5-dimethylcyclohex-2-enone

-
-
109-77-3
malononitrile

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium Tetrafluoroborate at 90℃; for 4h; | 98% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
39720-65-5
methoxycarbonylmethyltriphenylphosphonium iodide

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In tetrahydrofuran at 50℃; for 12h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With magnetic CoII(macrocyclic Schiff base ligand) immobilized on Fe3O4 nanoparticles In neat (no solvent) at 100℃; for 0.183333h; | 97% |

-
-
5447-87-0
2-(1-phenylethylidene)propanedinitrile

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
1189109-70-3
2-((E)-3-(4-chloro-2-nitrophenyl)-1-phenylallylidene)malononitrile

| Conditions | Yield |
|---|---|
| With malononitrile at 90℃; for 4h; Ionic liquid; | 96% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With diethylamino-sulfur trifluoride In dichloromethane at 20℃; for 3.33h; | 96% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
6336-44-3, 7547-96-8, 88982-39-2, 7547-97-9
(E)-propenylboronic acid

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; potassium phosphate monohydrate; palladium diacetate In tetrahydrofuran; water at 40℃; Suzuki Coupling; Inert atmosphere; | 95.8% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium Tetrafluoroborate at 80℃; for 0.5h; | 95% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
1445973-83-0
C35H28NO3PS

-
-
1445973-87-4
(E)-1-(2-(4-chloro-2-nitrostyryl)-1-(phenylsulfonyl)-1H-indol-3-yl)ethanone

| Conditions | Yield |
|---|---|
| In dichloromethane for 6h; Reflux; Inert atmosphere; | 94% |
| In dichloromethane for 6h; Wittig Olefination; Inert atmosphere; Reflux; | 94% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 70℃; for 1.5h; | 94% |

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; Heating / reflux; | 93% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
1586740-46-6
(Z)-methyl 2-bromo-3-(4-chloro-2-nitrophenyl)acrylate

| Conditions | Yield |
|---|---|
| Stage #1: methyl (triphenylphosphoranylidene)acetate With dimethylbromosulphonium bromide In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #2: 4-Chloro-2-nitrobenzaldehyde With triethylamine In dichloromethane at -78 - 20℃; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With nano cellulose OBF2 In ethanol at 78℃; for 0.25h; Green chemistry; | 92% |
| With nano-cellulose-OBF2 In ethanol at 78℃; for 0.25h; Green chemistry; | 92% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
127-17-3
2-oxo-propionic acid

-
-
406500-11-6
5-(4-chloro-2-nitro-phenyl)-3-hydroxy-5H-furan-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium hydroxide In neat (no solvent) at 20℃; for 0.166667h; Schlenk technique; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; Knoevenagel condensation; | 89.3% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium Tetrafluoroborate at 80℃; for 3h; | 88% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
2365-48-2
Methyl thioglycolate

-
-
104795-85-9
6-chlorobenzo[b]thiophene-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 24.5h; | 86% |
-
-
627-37-2
N-methyl-N-allylamine

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
807640-61-5
benzenemethanamine,4-chloro-N-methyl-2-nitro-N-2-propenyl-

| Conditions | Yield |
|---|---|
| Stage #1: N-methyl-N-allylamine; 4-Chloro-2-nitrobenzaldehyde In dichloromethane at 20℃; for 15h; Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; for 3.5h; | 86% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
6967-12-0
6-amino-1H-indazole

-
-
2749-59-9
1,3-dimethyl-5-pyrazolone

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 86% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
22996-18-5
(4-chloro-2-nitrophenyl)methanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; tetrabutylammomium bromide In benzene at 20 - 25℃; for 1h; | 85% |
| With sodium tetrahydroborate In methanol at 0 - 5℃; for 1h; Inert atmosphere; | 78% |
| With sodium tetrahydroborate In methanol at 20 - 30℃; for 1h; | 75% |
| With methanol; sodium hydroxide; formaldehyd | |
| With sodium tetrahydroborate In tetrahydrofuran at 20℃; for 1h; |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 85% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
153898-02-3
phenyl-3-<2-(1-pyrrolyl)thienyl>methylenimine

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; Heating; | 84% |
-
-
79-24-3
Nitroethane

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
188716-71-4
(E)-1-(4-chloro-2-nitrophenyl)-2-nitroprop-1-ene

| Conditions | Yield |
|---|---|
| With ammonium acetate at 60℃; for 4h; | 84% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
397328-47-1
(E)-3-(4-Chloro-2-nitro-phenyl)-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In toluene for 3h; Heating; | 84% |
-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
91-59-8
naphthalen-2-ylamine

-
-
29976-53-2
N-ethoxycarbonyl-4-piperidone

-
-
1206781-10-3
ethyl 1,2-dihydro-5-(4-chloro-2-nitrophenyl)naphtha [2,1-c][2,7]naphthyridine-3(4H)-carboxylate

| Conditions | Yield |
|---|---|
| With iodine In tetrahydrofuran for 10h; Reflux; | 84% |

-
-
5551-11-1
4-Chloro-2-nitrobenzaldehyde

-
-
172877-90-6
1-[2-(bromomethyl)-1-(phenylsulfonyl)-1H-indol-3-yl]propan-1-one

-
-
1558054-04-8
(E)-1-[2-(4-chloro-2-nitrostyryl)-1-(phenylsulfonyl)-1H-indol-3-yl]-propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-[2-(bromomethyl)-1-(phenylsulfonyl)-1H-indol-3-yl]propan-1-one With triphenylphosphine In tetrahydrofuran for 6h; Wittig Olefination; Inert atmosphere; Reflux; Stage #2: 4-Chloro-2-nitrobenzaldehyde With potassium carbonate In dichloromethane at 20℃; for 24h; Wittig Olefination; Inert atmosphere; | 84% |
| Stage #1: 1-[2-(bromomethyl)-1-(phenylsulfonyl)-1H-indol-3-yl]propan-1-one With triphenylphosphine In tetrahydrofuran for 6h; Wittig Olefination; Reflux; Inert atmosphere; Stage #2: 4-Chloro-2-nitrobenzaldehyde With potassium carbonate In dichloromethane at 20℃; for 24h; Wittig Olefination; Inert atmosphere; | 84% |
4-chloro-2-nitrobenzaldehyde Chemical Properties
The MF of 4-chloro-2-nitrobenzaldehyde(5551-11-1): C7H4ClNO3
The MW of 4-chloro-2-nitrobenzaldehyde(5551-11-1): 185.56
EINECS: 226-915-7
mp: 68 °C
Density: 1.485 g/cm3
Flash Point: 138.4 °C
Boiling Point: 305.2 °C at 760 mmHg
Index of Refraction: 1.63
Appearance: Yellow Powder
Categories: FINE Chemical & INTERMEDIATES;Aromatic Aldehydes & Derivatives (substituted);Aromatics;Aldehydes;C7;Carbonyl Compounds
Synonyms: 2-NITRO-4-CHLOROBENZALDEHYDE;4-CHLORO-2-NITROBENZALDEHYDE;4-chloro-2-nitrobenzaldehyde*crystalline
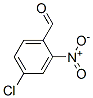
The Structure of 4-chloro-2-nitrobenzaldehyde(5551-11-1):
4-chloro-2-nitrobenzaldehyde Safety Profile
 Xi
Xi Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-37/39-36
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
37/39: Wear suitable gloves and eye/face protection
36: Wear suitable protective clothing
WGK Germany: 3
Related Products
- 4-chloro-2-nitrobenzaldehyde
- 5551-12-2
- 55512-05-5
- 55512-33-9
- 55512-82-8
- 55515-98-5
- 55-51-6
- 55516-54-6
- 555-16-8
- 55518-81-5
- 55520-20-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View