-
Name
Aminoglutethimide
- EINECS 204-756-4
- CAS No. 125-84-8
- Article Data9
- CAS DataBase
- Density 1.173 g/cm3
- Solubility slightly soluble in water
- Melting Point 152-154 °C(lit.)
- Formula C13H16N2O2
- Boiling Point 457.4 °C at 760 mmHg
- Molecular Weight 232.282
- Flash Point 230.4 °C
- Transport Information UN 3249
- Appearance white solid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Glutarimide,2-(p-aminophenyl)-2-ethyl- (6CI,7CI,8CI);(RS)-Aminoglutethimide;3-(4-Aminophenyl)-3-ethylpiperidine-2,6-dione;Ba-16038;Cytadren;DL-Aminoglutethimide;Elipten;NSC 330915;Orimeten;p-Aminoglutethimide;
- PSA 72.19000
- LogP 2.26320
Synthetic route

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 1h; | 99% |
-
-
38527-73-0
rac-3-ethyl-3-(4-nitrophenyl)piperidine-2,6-dione

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 760 Torr; for 1h; | 95% |
| With hydrogen; palladium on activated charcoal In ethanol | 91% |
| With hydrogen; palladium on activated charcoal In methanol for 5h; Ambient temperature; | 90% |
-
-
183663-77-6
1-hydroxy-3-ethyl-3-(4-aminophenyl)piperidine-2,6-dione

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine; α-bromoacetophenone In acetonitrile for 24h; Ambient temperature; |

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With nickel; ethyl acetate Hydrogenation; |

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 83 percent / trifluoroacetamide, 1-hydroxybenzotriazole, triethylamine, N-(3-dimethylamino)propyl-N-ethylcarbodiimide hydrochloride / 1 h / Ambient temperature 2: 95 percent / H2 / Pd/C / ethanol / 1 h / 760 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: 81 percent / N-ethyl-N-dimethylaminopropylcarbodiimide hydrochloride, 1-hydroxybenzotriazole, triethylamine / CH2Cl2 / 0 °C 2: 10percent Pd/C, H2 / methanol / 2 h 3: triethylamine, bromoacetophenone, DMAP / acetonitrile / 24 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) NaH / 1) DMF, 0 deg C, 60 min, 2) DMF, 0 deg C, 0.5 h 2: 60 percent / polyphosphoric acid / 0.5 h / 180 °C 3: 90 percent / H2 / Pd/C / methanol / 5 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 58 percent / NaH / dimethylformamide / 2 h / 0 °C 2: 1) NaH / 1) DMF, 0 deg C, 60 min, 2) DMF, 0 deg C, 0.5 h 3: 60 percent / polyphosphoric acid / 0.5 h / 180 °C 4: 90 percent / H2 / Pd/C / methanol / 5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: 65 percent / NaH / dimethylformamide / 2.5 h / 0 °C 2: 1) NaH / 1) DMF, 0 deg C, 60 min, 2) DMF, 0 deg C, 0.5 h 3: 60 percent / polyphosphoric acid / 0.5 h / 180 °C 4: 90 percent / H2 / Pd/C / methanol / 5 h / Ambient temperature View Scheme |
-
-
203808-68-8
4-(ethoxycarbonyl)-4-(4'-nitrophenyl)hexanonitrile

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / polyphosphoric acid / 0.5 h / 180 °C 2: 90 percent / H2 / Pd/C / methanol / 5 h / Ambient temperature View Scheme |
-
-
183663-75-4
1-benzyloxy-3-ethyl-3-(4-nitrophenyl)piperidine-2,6-dione

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 10percent Pd/C, H2 / methanol / 2 h 2: triethylamine, bromoacetophenone, DMAP / acetonitrile / 24 h / Ambient temperature View Scheme |
-
-
74220-50-1
2-ethyl-2-phenylglutarodinitrile

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 59 percent / concd H2SO4, CH3CO2H / 6 h 2: 9 percent / concd H2SO4, 63percent aq. HNO3 / 2 h / -10 - 0 °C 3: 91 percent / H2 / 10percent Pd/C / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 53 percent / trimethylbenzylammoniumhydroxide (Triton B) / dioxane; methanol / 15 h / Heating 2: 59 percent / concd H2SO4, CH3CO2H / 6 h 3: 9 percent / concd H2SO4, 63percent aq. HNO3 / 2 h / -10 - 0 °C 4: 91 percent / H2 / 10percent Pd/C / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 73 percent / NaNH2 / benzene; toluene / 1 h / 80 °C 2: 53 percent / trimethylbenzylammoniumhydroxide (Triton B) / dioxane; methanol / 15 h / Heating 3: 59 percent / concd H2SO4, CH3CO2H / 6 h 4: 9 percent / concd H2SO4, 63percent aq. HNO3 / 2 h / -10 - 0 °C 5: 91 percent / H2 / 10percent Pd/C / ethanol View Scheme |
-
-
77-21-4, 17575-58-5, 17575-59-6, 18389-24-7
Glutethimide

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 9 percent / concd H2SO4, 63percent aq. HNO3 / 2 h / -10 - 0 °C 2: 91 percent / H2 / 10percent Pd/C / ethanol View Scheme |

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol / 12 h / 20 °C 2: acetic acid / 1 h / 20 °C View Scheme |

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: tetrahydrofuran / 0.5 h / 20 °C / Molecular sieve 1.2: 2 h / 0 °C / Molecular sieve 2.1: methanol / 12 h / 20 °C 3.1: acetic acid / 1 h / 20 °C View Scheme |
-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; deuterium In tetrahydrofuran at 55℃; under 750.075 Torr; for 21h; Inert atmosphere; | 100% |
| With water-d2 In tetrahydrofuran at 80℃; under 760.051 Torr; for 3h; Sealed tube; | 82% |
-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With fluorosulfonyl azide; potassium hydrogencarbonate In tert-butyl methyl ether; water; N,N-dimethyl-formamide at 20℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile for 2h; Heating; | 93% |
-
-
125-84-8
aminoglutethimide

-
-
67393-83-3, 67393-84-4, 67663-02-9, 91603-21-3, 91603-22-4, 118759-60-7, 118759-61-8
2-((2RS,3RS)-3-ethyloxiran-2-yl)ethan-1-ol

| Conditions | Yield |
|---|---|
| With 3,4,5-trifluorophenylboronic acid at 60℃; for 24h; regioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| In water at 0℃; for 0.75h; Inert atmosphere; | 90% |


| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 40℃; for 4h; | 89% |
-
-
125-84-8
aminoglutethimide

-
-
68460-01-5
2,3,4,5-tetrabromo-6-sulfo-benzoic acid-anhydride

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile for 2h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| With pyridine for 1h; Heating; | 85.36% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 40℃; for 4h; | 85% |

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With sodium nitrite In water at 0℃; for 0.5h; | 84% |
-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; trifuran-2-yl-phosphane In dichloromethane at 20℃; for 12h; | 83% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 40℃; for 4h; | 82% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 82% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 82% |
-
-
125-84-8
aminoglutethimide

-
-
388628-24-8
1-[(dimethylamino)methylene]-1,3-dihydro-2H-pyrrolol[3,2-f]quinolin-2-one

-
-
297756-95-7
3-ethyl-3-(4-{(Z)-[(2-oxo-2,3-dihydro-1H-pyrrolo[3,2-f]quinolin-1-ylidene)methyl]amino}phenyl)-2,6-piperidinedione

| Conditions | Yield |
|---|---|
| 81% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetonitrile at 20℃; for 2h; | 80% |
-
-
95794-28-8
ethyl 3-bromomethyl-4-oxochromene-2-carboxylate

-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| In ethanol for 4h; Heating; | 78% |

| Conditions | Yield |
|---|---|
| In methanol for 2h; Heating; | 78% |

| Conditions | Yield |
|---|---|
| In methanol for 2h; Heating; | 77% |

| Conditions | Yield |
|---|---|
| With pyridine for 1h; Heating; | 76.96% |
-
-
125-84-8
aminoglutethimide

| Conditions | Yield |
|---|---|
| In methanol for 2h; Heating; | 76% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 40℃; for 4h; | 76% |

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In ethyl acetate at 70℃; for 12h; regioselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| With 9-(2-chlorophenyl)acridine; di-tert-butyl peroxide; copper(II) hexafluoroacetylacetonate In ethyl acetate at 35℃; for 36h; Inert atmosphere; Irradiation; | 76% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 75% |
-
-
125-84-8
aminoglutethimide

-
-
141109-47-9
[di(propan-2-yl)amino](oxo)acetyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: aminoglutethimide; [di(propan-2-yl)amino](oxo)acetyl chloride In dichloromethane at 0℃; for 0.166667h; Stage #2: With triethylamine In dichloromethane at 20℃; for 2h; | 75% |
Aminoglutethimide Specification
The Aminoglutethimide is an organic compound with the formula C13H16N2O2. The IUPAC name of this chemical is 3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione. With the CAS registry number 125-84-8, it is also named as 2,6-piperidinedione, 3-(4-aminophenyl)-3-ethyl-. The product's categories are Antitumors for Research and Experimental Use; Biochemistry; Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals. Besides, it is a white solid, which should be stored in a closed palce at temperature of 2 - 8 °C. It is used as an aromatase inhibitor and blocks adrenal steroidogenesis.
Physical properties about Aminoglutethimide are: (1)ACD/LogP: 1.41; (2)ACD/LogD (pH 5.5): 1.38; (3)ACD/LogD (pH 7.4): 1.41; (4)ACD/BCF (pH 5.5): 6.54; (5)ACD/BCF (pH 7.4): 6.98; (6)ACD/KOC (pH 5.5): 130.94; (7)ACD/KOC (pH 7.4): 139.87; (8)#H bond acceptors: 4; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 40.62 Å2; (12)Index of Refraction: 1.566; (13)Molar Refractivity: 64.58 cm3; (14)Molar Volume: 197.9 cm3; (15)Polarizability: 25.6×10-24cm3; (16)Surface Tension: 46.6 dyne/cm; (17)Density: 1.173 g/cm3; (18)Flash Point: 230.4 °C; (19)Enthalpy of Vaporization: 71.75 kJ/mol; (20)Boiling Point: 457.4 °C at 760 mmHg; (21)Vapour Pressure: 1.5E-08 mmHg at 25°C.
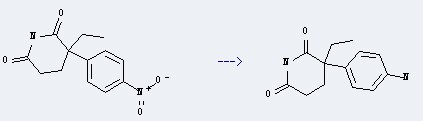
Preparation: this chemical can be prepared by 3-ethyl-3-(4-nitro-phenyl)-piperidine-2,6-dione. This reaction will need reagent H2, catalyst 10percent Pd/C and solvent ethanol. The yield is about 91%.
Uses of Aminoglutethimide: it can be used to produce o-iodoaminoglutethimide by heating. It will need reagent I2/KI and solvent methanol, H2O with reaction time of 30 min. The yield is about 70%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1NC(=O)CCC1(c2ccc(N)cc2)CC
(2)InChI: InChI=1/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
(3)InChIKey: ROBVIMPUHSLWNV-UHFFFAOYAT
(4)Std. InChI: InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
(5)Std. InChIKey: ROBVIMPUHSLWNV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | LDLo | oral | 21mg/kg/3D-I (21mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Annals of Internal Medicine. Vol. 105, Pg. 633, 1986. |
| mouse | LD50 | intraperitoneal | 625mg/kg (625mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ANTIPSYCHOTIC | Journal of Medicinal Chemistry. Vol. 18, Pg. 736, 1975. |
| women | TDLo | oral | 20500mg/kg/94 (20500mg/kg) | BLOOD: AGRANULOCYTOSIS | British Medical Journal. Vol. 291, Pg. 970, 1985. |
Related Products
- Aminoglutethimide
- Aminoglutethimide phosphate
- 125849-94-7
- 12585-31-8
- 125867-25-6
- 125867-31-4
- 125872-95-9
- 1258-84-0
- 125903-81-3
- 125904-11-2
- 125911-68-4
- 125917-60-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View