-
Name
N-AMYL NITRATE
- EINECS 213-684-2
- CAS No. 1002-16-0
- Article Data12
- CAS DataBase
- Density 1.023 g/cm3
- Solubility Insoluble in water
- Melting Point -123.2°C
- Formula C5H11NO3
- Boiling Point 157.498 °C at 760 mmHg
- Molecular Weight 133.147
- Flash Point 56.753 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Pentylnitrate (6CI,7CI);1-Pentyl nitrate;n-Pentyl nitrate;
- PSA 55.05000
- LogP 1.90810
Amyl nitrate Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
Amyl nitrate Specification
The Amyl nitrate, with the CAS registry number 1002-16-0, is also known as Nitric acid, pentyl ester. Its EINECS registry number is 213-684-2. This chemical's molecular formula is C5H11NO3 and molecular weight is 133.15. What's more, both its IUPAC name and systematic name are the same which is called Pentyl nitrate. It should be stored in a cool, dry and well-ventilated place. Amyl nitrate are employed as reagents in organic synthesis. It is used as an additive in diesel fuel, where it acts as an 'ignition improver' by accelerating the ignition of fuel.
Physical properties about Amyl nitrate are: (1)ACD/LogP: 3.01; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.01; (4)ACD/LogD (pH 7.4): 3.01; (5)ACD/BCF (pH 5.5): 114.27; (6)ACD/BCF (pH 7.4): 114.27; (7)ACD/KOC (pH 5.5): 1034.39; (8)ACD/KOC (pH 7.4): 1034.39; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 55.05 Å2; (13)Index of Refraction: 1.42; (14)Molar Refractivity: 32.981 cm3; (15)Molar Volume: 130.21 cm3; (16)Polarizability: 13.075×10-24cm3; (17)Surface Tension: 31.833 dyne/cm; (18)Density: 1.023 g/cm3; (19)Flash Point: 56.753 °C; (20)Enthalpy of Vaporization: 37.795 kJ/mol; (21)Boiling Point: 157.498 °C at 760 mmHg; (22)Vapour Pressure: 3.551 mmHg at 25 °C.
Preparation of Amyl nitrate: this chemical can be prepared by 1-nitrooxy-pentane. The research objective is Mechanism//Kinetics. This reaction needs solvent 1,2,3,4-tetrahydro-naphthalene at temperature of 154 °C. The yield is 98 %.
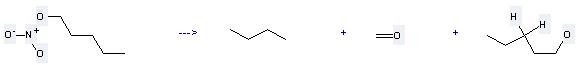
Uses of Amyl nitrate: it is used to produce other chemicals. For example, it can produce 1-nitrooxy-pentane. This reaction needs reagents KNO3, BF3*1.25H2O solvent CH2Cl2 at ambient temperature. The reaction time is 2 hours. The yield is 75 %.

You can still convert the following datas into molecular structure:
(1) SMILES: [O-][N+](=O)OCCCCC
(2) InChI: InChI=1S/C5H11NO3/c1-2-3-4-5-9-6(7)8/h2-5H2,1H3
(3) InChIKey: HSNWZBCBUUSSQD-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LCLo | inhalation | 1703ppm (1703ppm) | LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | AMA Archives of Industrial Health. Vol. 11, Pg. 290, 1955. |
| mouse | LCLo | inhalation | 1807ppm/7H (1807ppm) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | AMA Archives of Industrial Health. Vol. 11, Pg. 290, 1955. |
| rabbit | LCLo | inhalation | 1807ppm/7H (1807ppm) | LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | AMA Archives of Industrial Health. Vol. 11, Pg. 290, 1955. |
Related Products
- Amyl 2-methylbutyrate
- Amyl acetate
- Amyl cinnamic acetate
- AMYL CINNAMYLIDENE METHYL ANTHRANILATE
- Amyl isoeugenol
- Amyl nitrate
- Amyl phthalate
- Amyl propanoate
- Amyl salicylate
- Amylamine
- 10021-67-7
- 1002-19-3
- 100-22-1
- 10022-13-6
- 10022-28-3
- 10022-31-8
- 100224-74-6
- 10022-66-9
- 10022-68-1
- 1002-28-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View