-
Name
(+/-)-2-ACETYL-9-AZA BICYCLO[4.2.1]NON-2-ENE FUMARATE
- EINECS
- CAS No. 64285-06-9
- Article Data11
- CAS DataBase
- Density 1.037 g/cm3
- Solubility Soluble to 50 mM in Water
- Melting Point
- Formula C10H15 N O
- Boiling Point 291 °C at 760 mmHg
- Molecular Weight 165.235
- Flash Point 124.7 °C
- Transport Information
- Appearance Lyophilized Solid
- Safety Poison by intraperitoneal route. An experimental teratogen. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ethanone,1-(9-azabicyclo[4.2.1]non-2-en-2-yl)-, (1R)-; 9-Azabicyclo[4.2.1]nonane,ethanone deriv.; (+)-Anatoxin a; (+)-Anatoxin a; (+)-Anatoxin-a; Anatoxin I; Anatoxin a; Anatoxin a
- PSA 29.10000
- LogP 1.74500
Synthetic route
-
-
120692-41-3
(+)-(1R)-2-acetyl-9-benzyloxycarbonyl-9-azabicyclo[4.2.1]-2-nonene

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide In acetonitrile at -10℃; for 0.333333h; | 99% |
-
-
143264-77-1
(-)-N-tosylanatoxin-a

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; sodium amalgam In methanol at -40℃; for 0.166667h; | 76% |
-
-
216166-93-7
(1R,6R)-2-Acetyl-9-aza-bicyclo[4.2.1]non-2-ene-9-carboxylic acid vinyl ester

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| With hydrochloric acid diethyl ether In ethanol at 50℃; |
-
-
64700-65-8
methyl (2R)-pyroglutamate

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 100 percent / LiHMDS / tetrahydrofuran / -78 - 20 °C 2.1: Mg / tetrahydrofuran / 0.83 h / 20 °C 2.2: 73 percent / TMEDA / tetrahydrofuran / 1.5 h / -78 °C 3.1: BF3*OEt2; Ph3SiH / CH2Cl2 / 2.5 h / -78 - 20 °C 3.2: DIBAL-H / toluene / 3 h / -78 °C 3.3: 826 mg / Cs2CO3 / toluene; propan-2-ol / 17 h / 20 °C 4.1: 97 percent / NaHMDS / tetrahydrofuran / 2 h / -78 °C 5.1: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 6.1: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 7.1: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 8.1: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-34-8
1-benzyl 2-methyl (R)-5-oxopyrrolidine-1,2-dicarboxylate

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: Mg / tetrahydrofuran / 0.83 h / 20 °C 1.2: 73 percent / TMEDA / tetrahydrofuran / 1.5 h / -78 °C 2.1: BF3*OEt2; Ph3SiH / CH2Cl2 / 2.5 h / -78 - 20 °C 2.2: DIBAL-H / toluene / 3 h / -78 °C 2.3: 826 mg / Cs2CO3 / toluene; propan-2-ol / 17 h / 20 °C 3.1: 97 percent / NaHMDS / tetrahydrofuran / 2 h / -78 °C 4.1: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 5.1: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 6.1: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 7.1: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-37-1
(2R,5R)-5-but-3-enyl-2-ethynylpyrrolidine-1-carboxylic acid 1-benzyl ester

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 97 percent / NaHMDS / tetrahydrofuran / 2 h / -78 °C 2: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 3: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 4: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 5: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-38-2
(2R)-but-3-enyl-(5R)-prop-1-ynylpyrrolidine-1-carboxylic acid 1-benzyl ester

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 2: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 3: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 4: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-39-3
(+)-(1R)-2-isopropenyl-9-benzyloxycarbonyl-9-azabicyclo[4.2.1]-2-nonene

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 2: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 3: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-35-9
(2R)-benzyloxycarbonylamino-5-oxonon-8-enoic acid methyl ester

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: BF3*OEt2; Ph3SiH / CH2Cl2 / 2.5 h / -78 - 20 °C 1.2: DIBAL-H / toluene / 3 h / -78 °C 1.3: 826 mg / Cs2CO3 / toluene; propan-2-ol / 17 h / 20 °C 2.1: 97 percent / NaHMDS / tetrahydrofuran / 2 h / -78 °C 3.1: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 4.1: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 5.1: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 6.1: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
690211-40-6
(+)-(1R)-2-(1,2-dihydroxy-1-methylethyl)-9-benzyloxycarbonyl-9-azabicyclo[4.2.1]-2-nonene

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 2: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
760968-59-0
(2R)-but-3-enyl-(5R)-2-(1-methylsiletan-1-ylethynyl)pyrrolidine-1-carboxylic acid benzyl ester

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 9 percent / second generation Grubbs catalyst / CH2Cl2 / 80 °C 2: 97 percent / NaHMDS / tetrahydrofuran / 2 h / -78 °C 3: 84 percent / second generation Grubbs catalyst / CH2Cl2 / 16 h 4: 76 percent / Et3N; OsO4 / tetrahydrofuran / 22 h / -78 - 20 °C 5: 95 percent / NaIO4 / tetrahydrofuran; H2O / 1.5 h / 20 °C 6: 99 percent / TMSI / acetonitrile / 0.33 h / -10 °C View Scheme |
-
-
126811-06-1
(1R)-9-methyl-9-azabicyclo[4.2.1]nonan-2-one

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: LiN(SiMe3)2 / tetrahydrofuran / -40 °C 2: 95 percent / K2CO3 / CH2Cl2 / Heating 3: AlCl3 / 1,2-dichloro-ethane / -10 °C 4: 65 percent / LiBr, Li2CO3 / dimethylformamide / 150 °C 5: HCl*Et2O / ethanol / 50 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / K2CO3 / CH2Cl2 / Heating 2: AlCl3 / 1,2-dichloro-ethane / -10 °C 3: 65 percent / LiBr, Li2CO3 / dimethylformamide / 150 °C 4: HCl*Et2O / ethanol / 50 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / LiBr, Li2CO3 / dimethylformamide / 150 °C 2: HCl*Et2O / ethanol / 50 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: AlCl3 / 1,2-dichloro-ethane / -10 °C 2: 65 percent / LiBr, Li2CO3 / dimethylformamide / 150 °C 3: HCl*Et2O / ethanol / 50 °C View Scheme |
-
-
132974-84-6
(-)-(5S)-5-{[(tert-butyldiphenylsilyl)oxy]methyl}-1-tosylpyrrolidin-2-one

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 92 percent / DIBAL / CH2Cl2 / -78 °C 2: 87 percent / PPTS / methanol 3: 96 percent / Bu4NF / tetrahydrofuran 4: 1.) BuLi / 1.) THF, -78 deg C 5: toluene / Irradiation 6: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 7: 79 percent / NaH / tetrahydrofuran 8: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 9: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 12 steps 1: 92 percent / DIBAL / CH2Cl2 / -78 °C 2: 87 percent / PPTS / methanol 3: 96 percent / Bu4NF / tetrahydrofuran 4: 1.) BuLi / 1.) THF, -78 deg C 5: toluene / Irradiation 6: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 7: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 8: tetrahydrofuran 9: 76 percent / TiCl4 / CH2Cl2 / -78 °C 10: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 11: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 12: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-74-8
[(2S,5R)-5-Methoxy-1-(toluene-4-sulfonyl)-pyrrolidin-2-yl]-methanol

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1.) BuLi / 1.) THF, -78 deg C 2: toluene / Irradiation 3: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 4: 79 percent / NaH / tetrahydrofuran 5: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 6: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 9 steps 1: 1.) BuLi / 1.) THF, -78 deg C 2: toluene / Irradiation 3: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 4: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 5: tetrahydrofuran 6: 76 percent / TiCl4 / CH2Cl2 / -78 °C 7: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 8: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 9: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-75-9
(2R,5R)-2-But-3-enyl-5-methoxy-1-(toluene-4-sulfonyl)-pyrrolidine

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 2: 79 percent / NaH / tetrahydrofuran 3: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 4: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 7 steps 1: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 2: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 3: tetrahydrofuran 4: 76 percent / TiCl4 / CH2Cl2 / -78 °C 5: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 6: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 7: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-80-6
(1R,6R)-9-(Toluene-4-sulfonyl)-2-vinyl-9-aza-bicyclo[4.2.1]nonane

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 2: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 3: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 79 percent / NaH / tetrahydrofuran 2: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 3: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-78-2
4-[(2R,5R)-5-Methoxy-1-(toluene-4-sulfonyl)-pyrrolidin-2-yl]-butan-1-ol

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 2: tetrahydrofuran 3: 76 percent / TiCl4 / CH2Cl2 / -78 °C 4: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 5: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 6: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 2: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: tetrahydrofuran 2: 76 percent / TiCl4 / CH2Cl2 / -78 °C 3: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 4: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 5: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-76-0, 143292-29-9
(E)-6-[(2R,5R)-5-Methoxy-1-(toluene-4-sulfonyl)-pyrrolidin-2-yl]-hex-3-en-2-one

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 2: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-79-3, 143292-31-3
(2R,5R)-2-Methoxy-1-(toluene-4-sulfonyl)-5-((E)-5-trimethylsilanyl-pent-4-enyl)-pyrrolidine

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 76 percent / TiCl4 / CH2Cl2 / -78 °C 2: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 3: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 4: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / Irradiation 2: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 3: 79 percent / NaH / tetrahydrofuran 4: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 5: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 8 steps 1: toluene / Irradiation 2: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 3: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 4: tetrahydrofuran 5: 76 percent / TiCl4 / CH2Cl2 / -78 °C 6: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 7: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 8: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-72-6, 143292-32-4
N-p-toluenesulphonyl(5-diphenyl-tert-butylsilyloxymethyl)-2-hydroxypyrrolidine

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 87 percent / PPTS / methanol 2: 96 percent / Bu4NF / tetrahydrofuran 3: 1.) BuLi / 1.) THF, -78 deg C 4: toluene / Irradiation 5: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 6: 79 percent / NaH / tetrahydrofuran 7: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 8: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 11 steps 1: 87 percent / PPTS / methanol 2: 96 percent / Bu4NF / tetrahydrofuran 3: 1.) BuLi / 1.) THF, -78 deg C 4: toluene / Irradiation 5: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 6: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 7: tetrahydrofuran 8: 76 percent / TiCl4 / CH2Cl2 / -78 °C 9: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 10: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 11: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
-
-
143264-73-7, 143292-30-2
N-p-toluenesulphonyl(5-diphenyl-tert-butylsilyloxymethyl)-2-methoxypyrrolidine

-
-
64285-06-9
anatoxin-a

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 96 percent / Bu4NF / tetrahydrofuran 2: 1.) BuLi / 1.) THF, -78 deg C 3: toluene / Irradiation 4: 1.) O3, 2.) Me2S / 1.) CH2Cl2, -78 deg C 5: 79 percent / NaH / tetrahydrofuran 6: 1.) HCl, 2.) DBU / 1.) CH2Cl2, MeOH, 2.) toluene, reflux 7: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme | |
| Multi-step reaction with 10 steps 1: 96 percent / Bu4NF / tetrahydrofuran 2: 1.) BuLi / 1.) THF, -78 deg C 3: toluene / Irradiation 4: 1.) BH3*DMS, 2.) H2O2, 6M NaOH / 1.) THF 5: 1.) (COCl)2, 2.) Et3N / 1.) DMSO, 2.) CH2Cl2, -78 deg C 6: tetrahydrofuran 7: 76 percent / TiCl4 / CH2Cl2 / -78 °C 8: 71 percent / O2 / PdCl2, CuCl / dimethylformamide; H2O 9: 1.) KH, TMSCl, 2.) Et3N / 2.) Pd(OAc)2 / 2.) CH3CN 10: 76 percent / Na(Hg), Na2HPO4 / methanol / 0.17 h / -40 °C View Scheme |
Anatoxin I Chemical Properties
IUPAC Name: 1-[(1R,6R)-9-Azabicyclo[4.2.1]non-4-en-5-yl]ethanone
Synonyms: (1R)-ethanon;anatoxini ; (+/-)-Anatoxin a ; (+/-)-Anatoxin a fumarate ; (+/-)-Anatoxin a fumerate ; (+/-)-2-Acetyl-9-aza bicyclo[4.2.1]non-2-ene fumarate ; (-)-Anatoxin ; (1β,6β)-5-Acetyl-9-azabicyclo[4.2.1]non-4-ene
Product Categories: Acetylcholine receptor
CAS NO: 64285-06-9
Molecular Formula of Anatoxin I (CAS NO.64285-06-9) : C10H15NO
Molecular Weight of Anatoxin I (CAS NO.64285-06-9) : 165.23
Molecular Structure of Anatoxin I (CAS NO.64285-06-9) : 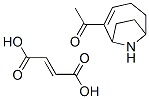
Mol File: 64285-06-9.mol
Index of Refraction: 1.503
Surface Tension: 34 dyne/cm
Density: 1.037 g/cm3
Flash Point: 124.7 °C
Enthalpy of Vaporization: 53.04 kJ/mol
Boiling Point: 291 °C at 760 mmHg
Vapour Pressure: 0.002 mmHg at 25°C
Anatoxin I Toxicity Data With Reference
| 1. | ipr-mus LDLo:250 µg/kg | TOXIA6 Toxicon. 27 (1989),79. |
Anatoxin I Safety Profile
Poison by intraperitoneal route. An experimental teratogen. When heated to decomposition it emits toxic fumes of NOx.
Anatoxin I Specification
Category: Toxic Substances
Toxicity classification: highly toxic
Acute toxicity: abdominal - mice LDL0: 0.25 mg / kg
Flammable hazardous characteristics: flammable; burning of toxic nitrogen oxide fumes
Storage features: Treasury ventilation, low temperature, dry
Fire-extinguishing agents: water, carbon dioxide, dry powder, sand
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
