-
Name
POTASSIUM ARSENATE
- EINECS
- CAS No. 7784-41-0
- Article Data28
- CAS DataBase
- Density 2.867
- Solubility slightly
- Melting Point 288
- Formula AsH3 O4 . K
- Boiling Point
- Molecular Weight 180.033
- Flash Point
- Transport Information
- Appearance
- Safety Confirmed human carcinogen. Mutation data reported. When heated to decomposition it emits toxic fumes of arsenic. See also ARSENIC COMPOUNDS.
- Risk Codes 45-23/25-50/53
-
Molecular Structure
- Hazard Symbols Toxic by ingestion and inhalation, strong irritant.
- Synonyms Arsenicacid (H3AsO4), monopotassium salt (8CI,9CI); Potassium arsenate (KH2AsO4)(6CI,7CI); Dihydrogen potassium arsenate; Hydrogen potassium arsenate(H2KAsO4); Macquer's salt; Monopotassium arsenate; Monopotassium dihydrogenarsenate; Potassium acid arsenate; Potassium dihydrogen arsenate; Potassiumdihydrogen arsenate (KH2AsO4); Potassium dihydrogen orthoarsenate; Potassiumhydrogen arsenate (KH2AsO4)
- PSA 80.59000
- LogP -1.73240
Arsenic acid, monopotassium salt Chemical Properties
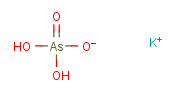
IUPAC Name: potassium dihydrogen arsorate
Molecular Weight: 180.03338 g/mol
Molecular Formula: AsH2KO4
H-Bond Donor: 2
H-Bond Acceptor: 4
Melting Point: 277-283 °C(lit.)
Density: 2.867
Solubility: clear to very slightly hazy, colorless to faintly yellow
Appreance: white to off-white powder
Merck: 13,7689
Exact Mass: 179.880612
MonoIsotopic Mass: 179.880612
Topological Polar Surface Area: 80.6
Heavy Atom Count: 6
EINECS: 232-065-8
Complexity: 61.9
Canonical SMILES: O[As](=O)(O)[O-].[K+]
InChI: InChI=1S/AsH3O4.K/c2-1(3,4)5;/h(H3,2,3,4,5);/q;+1/p-1
InChIKey: GVPLVOGUVQAPNJ-UHFFFAOYSA-M
Synonyms of Arsenic acid, monopotassium salt (CAS NO.7784-41-0): Arsenic acid potassium salt ; Potassium dihydrogen arsenate ; Potassium arsenate ; Potassium arsenate monobasic ; Arsenic acid potassium ; Monopotassium arsenate ; Potassium arsenate monobasic anhydrous ; Potassium dihydrogenarsenate , 99.9+%
Arsenic acid, monopotassium salt Uses
Arsenic acid, monopotassium salt (CAS NO.7784-41-0) is used to be pesticides, leather preservation, reagent, optical, electronic materials and so on.
Arsenic acid, monopotassium salt Consensus Reports
IARC Cancer Review: Human Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of chemicals to Man . 23 (1980),p. 39.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory. ARSENIC and its compounds are on the Community Right-To-Know List.
Arsenic acid, monopotassium salt Safety Profile
Confirmed human carcinogen. Mutation data reported. When heated to decomposition it emits toxic fumes of ARSENIC. See also ARSENIC COMPOUNDS.
Hazard Codes:  T,
T,  N
N
Risk Statements: 45-23/25-50/53
R45: May cause cancer
R23/25: Toxic by inhalation and if swallowed
R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
Safety Statements: 53-45-60-61
S53: Avoid exposure - obtain special instructions before use
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible)
S60: This material and its container must be disposed of as hazardous waste
S61: Avoid release to the environment. Refer to special instructions / safety data sheets
RIDADR: UN 1677 6.1/PG 2
WGK Germany: 3
RTECS: CG1100000
HazardClass: 6.1(a)
PackingGroup: II
Arsenic acid, monopotassium salt Standards and Recommendations
OSHA PEL: TWA 0.01 mg(As)/m3; Cancer Hazard
ACGIH TLV: TWA 0.01 mg/m3; Confirmed Human Carcinogen; BEI: 35 μ (As)/L inorganic arsenic and methylated metabolites in urine
NIOSH REL: (Arsenic, Inorganic) CL 2 µg(As)/m3/15 M
DOT Classification: 6.1; Label: Poison
Arsenic acid, monopotassium salt Specification
Arsenic acid, monopotassium salt (CAS NO.7784-41-0) is high toxic. It is noncombustible and it will decomposition of toxic arsenic fumes when heating. So the storage environment should be ventilate, low-temperature and dry.
Related Products
- Arsenic
- Arsenic (III) selenide
- Arsenic (III) sulfide
- Arsenic acid (H<sub>3</sub>AsO<sub>4</sub>),strontium salt (2:3)
- Arsenic acid (H3AsO4),calcium salt (2:3)
- Arsenic acid (H3AsO4),copper salt (1:?)
- Arsenic acid, aniline salt
- Arsenic acid, disodium salt
- Arsenic acid, disodium salt, hepta hydrate
- Arsenic acid, hemihydrate
- 7784-42-1
- 7784-46-5
- 7784-67-0
- 77847-30-4
- 77847-85-9
- 7784-82-9
- 778-48-3
- 77-85-0
- 7785-20-8
- 7785-21-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View