-
Name
ATROPINE SULFATE
- EINECS 200-235-0
- CAS No. 73791-47-6
- Article Data1
- CAS DataBase
- Density
- Solubility
- Melting Point 189-192 °C (A)(lit.)
- Formula C34H52N2O12S
- Boiling Point 429.8 °C at 760 mmHg
- Molecular Weight 405.469
- Flash Point 213.7 °C
- Transport Information UN 1544 6
- Appearance
- Safety 23-45
- Risk Codes 26/28
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms ATROPINE SULFATE, ATROPA BELLADONNA (LINNAEUS);ATROPINE SULPHATE
- PSA 132.75000
- LogP 2.29680
Atropine sulfate Chemical Properties
Molecular Formula: C34H52N2O12S
Molar mass: 712.8479 g/mol
Flash Point: 213.7 °C
Melting point: 189-192 °C (A)(lit.)
Boiling Point: 429.8 °C at 760 mmHg
Vapour Pressure: 3.76E-08 mmHg at 25°C
Structure of Atropine sulfate (73791-47-6):
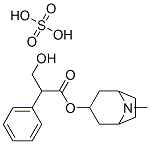
H-Bond Donor: 6
H-Bond Acceptor: 14
SMILES: O=S(=O)(O)O.O=C(OC2CC1N(C)C(CC1)C2)C(c3ccccc3)CO.O=C(OC2CC1N(C)C(CC1)C2)C(c3ccccc3)CO.O.O
InChI: InChI=1/2C17H23NO3.H2O4S.2H2O/c2*1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12;1-5(2,3)4;;/h2*2-6,13-16,19H,7-11H2,1H3;(H2,1,2,3,4);2*1H2
InChIKey: BXSVDJUWKSRQMD-UHFFFAOYAG
Std. InChI: InChI=1S/2C17H23NO3.H2O4S.2H2O/c2*1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12;1-5(2,3)4;;/h2*2-6,13-16,19H,7-11H2,1H3;(H2,1,2,3,4);2*1H2
Std. InChIKey: BXSVDJUWKSRQMD-UHFFFAOYSA-N
Atropine sulfate History
Mandragora (mandrake) was described by Theophrastus in the fourth century B.C. for treatment of gout, wounds, and sleeplessness, and as a love potion. By the first century A.D. Dioscorides recognized wine of mandrake as an anaesthetic for treatment of pain or sleeplessness, to be given prior to surgery or cautery. Atropine sulfate (73791-47-6) extracts from the Egyptian henbane were used by Cleopatra in the last century B.C. to dilate her pupils, in the hope that she would appear more alluring. In the Renaissance, women used the juice of the berries of Atropa belladonna to enlarge the pupils of their eyes, for cosmetic reasons; "bella donna" is Italian for "beautiful lady". The mydriatic effects of atropine were studied among others by the German chemist Friedrich Ferdinand Runge (1795–1867). In 1831, the pharmacist Mein succeeded the pure crystalline isolation of atropine. The substance was first synthesized by German chemist Richard Willstätter in 1901.
Atropinic shock therapy is an old and rarely-used method. Atropinic shock treatment is considered safe with careful monitoring and preparation, but it entails prolonged coma and it has many unpleasant side-effects, such as blurred vision.
Atropine sulfate Safety Profile
Hazard Codes: T+![]()
Risk Statements:
26: Very Toxic by inhalation
28: Very Toxic if swallowed
Safety Statements:
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
Atropine sulfate Specification
Atropine sulfate (73791-47-6), which also can be called for Benzeneacetic acid, alpha-(hydroxymethyl)- (3-endo)-8-methyl-8-azabicyclo(3.2.1)oct-3-yl ester, sulfate (2:1) (salt), dihydrate ; Egacene (TN) , is a tropane alkaloid extracted from deadly nightshade , jimsonweed , mandrake and other plants of the family Solanaceae. It serves as a drug with a wide variety of effects and is a competitive antagonist for the muscarinic acetylcholine receptor. And, it is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system. Being potentially deadly, it derives its name from Atropos, one of the three Fates who, according to Greek mythology, chose how a person was to die. Atropine is a racemic mixture of D-hyoscyamine and L-hyoscyamine, with most of its physiological effects due to L-hyoscyamine. Its pharmacological effects are due to binding to muscarinic acetylcholine receptors. It is an antimuscarinic agent.
Atropine sulfate (73791-47-6) is found in many members of the solanaceae family. The most commonly-found sources are Datura inoxia, Atropa belladonna, D. metel, and D. stramonium. The Nicotiana genus (including the tobacco plant, N. tabacum) is also found in the Solanaceae family, but these plants do not contain atropine or other tropane alkaloids.
Atropine sulfate (73791-47-6) increases firing of the sinoatrial node (SA) and conduction through the atrioventricular node (AV) of the heart, opposes the actions of the vagus nerve acetylcholine receptor sites, blocks, and decreases bronchial secretions.In general, atropine lowers the parasympathetic activity of all muscles and glands regulated by the parasympathetic nervous system. This occurs because atropine is a competitive antagonist of the muscarinic acetylcholine receptors . Therefore, it may cause swallowing difficulties and reduced secretions.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View