-
Name
Barbituric acid
- EINECS 200-658-0
- CAS No. 67-52-7
- Article Data96
- CAS DataBase
- Density 1.455 g/cm3
- Solubility 142 g/L (20 °C) in water
- Melting Point 248-252 °C (dec.)(lit.)
- Formula C4H4N2O3
- Boiling Point 260 °C
- Molecular Weight 128.087
- Flash Point 150 °C
- Transport Information
- Appearance cream coloured fine crystalline powder
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Hexahydropyrimidine-2,4,6-trione;2,4,6-Pyrimidinetrione;Barbitursaeure;Hydrouracil, 6-hydroxy-;2,4,6-Trihydroxypyrimidine(Barbituric acid);2, 4, 6-Trihydroxypyrimidine;Malonylurea;6-Hydroxyuracil;Urea, N,N-(1,3-dioxo-1,3-propanediyl)-;2,4,6-Pyrimidinetriol;pyrimidine-2,4,6(1H,3H,5H)-trione;2,4,6 (1H,3H,5H)-Pyrimidinetrione;Barbituric acid (VAN) (8CI);1,3-diazinane-2,4,6-trione;Pyrimidinetrione;sodium 4,6-dioxo-1H-pyrimidin-2-olate;Urea, N, N-(1,3-dioxo-1,3-propanediyl)-;2,4,6-Trioxohexahydropyrimidine;2,4,6-(1H,3H,5H)-Pyrimidinetrione;Malonylharnstoff;Pyrimidinetriol;1,2,3,4,5,6-Hexahydro-2,4,6-pyrimidinetrione;Barbiturate;2,4,6-Trihydroxypyrimidine;
- PSA 75.27000
- LogP -0.59990
Barbituric acid Chemical Properties
IUPAC Name: 1,3-diazinane-2,4,6-trione
Empirical Formula: C4H4N2O3
Molecular Weight: 128.0862g/mol
EINECS: 200-658-0
Structure of Barbituric acid (CAS NO.67-52-7):
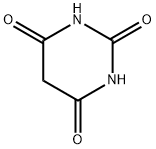
Melting Point: 248-252 °C (dec.)(lit.)
Boiling Point: 260 °C
Flash Point: 150 °C
Water Solubility: 142 g/L (20 ºC)
Index of Refraction: 1.492
Molar Refractivity: 25.53 cm3
Molar Volume: 88 cm3
Polarizability: 10.12×10-24cm3
Surface Tension: 48.7 dyne/cm
Density: 1.455 g/cm3
Product Categories: Pharmaceutical Intermediates;PYRIMIDINE;Miscellaneous Biochemicals;Pyrimidine series
Canonical SMILES: C1C(=O)NC(=O)NC1=O
InChI: InChI=1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9)
InChIKey: HNYOPLTXPVRDBG-UHFFFAOYSA-N
Barbituric acid History
Barbituric acid (CAS NO.67-52-7) was discovered by the German chemist Adolf von Baeyer on 4. December 1864—the feast of St Barbara and therefore the name given to the compound—by combining urea and malonic acid in a condensation reaction. and The first to be used in medicine was barbital (Veronal) starting in 1903, and the second, phenobarbitone a.k.a. phenobarbital was first marketed in 1912.
Barbituric acid Uses
Barbituric acid (CAS NO.67-52-7) is used in synthesis of riboflavin.
Barbituric acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 505mg/kg (505mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 106, Pg. 504, 1986. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. |
Barbituric acid Safety Profile
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
WGK Germany: 1
RTECS: CP8000000
HS Code: 29335200
Barbituric acid Specification
Barbituric acid , its cas register number is 67-52-7. It also can be called 2,4,6(1H,3H,5H)-Pyrimidinetrione ; 2,4,6-Pyrimidinetriol ; 2,4,6-Pyrimidinetrione ; 2,4,6-Trihydroxypyrimidine ; 2,4,6-Trioxohexahydropyrimidine ; 6-Hydroxyuracil ;
Hydrouracil, 6-hydroxy- ; Malonylurea ; Pyrimidinetriol ; Pyrimidinetrione ; Urea, N,N'-(1,3-dioxo-1,3-propanediyl)- . Barbituric acid (CAS NO.67-52-7) is a cream coloured fine crystalline powder.
Related Products
- Barbituric acid
- Barbituric acid, 5-ethyl-5-pentyl-
- 67528-13-6
- 67531-68-4
- 67531-86-6
- 67535-70-0
- 6753-62-4
- 67537-70-6
- 6754-13-8
- 67542-72-7
- 67544-71-2
- 6754-58-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View