-
Name
Benzanthrone
- EINECS 201-393-3
- CAS No. 82-05-3
- Article Data72
- CAS DataBase
- Density 1.286 g/cm3
- Solubility Insoluble in water, soluble in alcohol and other organic solvents
- Melting Point 170 °C
- Formula C17H10O
- Boiling Point 436.2 °C at 760 mmHg
- Molecular Weight 230.266
- Flash Point 196.1 °C
- Transport Information
- Appearance Light yellow powder
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,9-Benz-10-anthrone;7-Oxobenz[de]anthracene;Benzanthrenone;Benzoanthrone;NSC 5189;NSC 631641;Naphthanthrone;CCRIS 3173;Benz(de)anthracen-7-one;
- PSA 17.07000
- LogP 4.05120
Benzanthrone Consensus Reports
Reported in EPA TSCA Inventory.
Benzanthrone Specification
The Benzanthrone with CAS registry number of 82-05-3 is also known as Benz(de)anthracen-7-one. The IUPAC name is Benzo[a]phenalen-7-one. It belongs to product categories of Intermediates of Dyes and Pigments. Its EINECS registry number is 201-393-3. In addition, the formula is C17H10O and the molecular weight is 230.26. Besides. it is used as dye intermediate.
Physical properties about Benzanthrone are: (1)ACD/LogP: 4.81; (2)ACD/LogD (pH 5.5):; (3)4.81 ACD/LogD (pH 7.4): 4.81; (4)ACD/BCF (pH 5.5): 2667.48; (5)ACD/BCF (pH 7.4): 2667.48; (6)ACD/KOC (pH 5.5): 9862.76; (7)ACD/KOC (pH 7.4): 9862.76; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.734; (10)Molar Refractivity: 71.76 cm3; (11)Molar Volume: 178.9 cm3; (12)Surface Tension: 58.3 dyne/cm; (13)Density: 1.286 g/cm3; (14)Flash Point: 196.1 °C; (15)Enthalpy of Vaporization: 69.26 kJ/mol; (16)Boiling Point: 436.2 °C at 760 mmHg; (17)Vapour Pressure: 8.27E-08 mmHg at 25 °C.
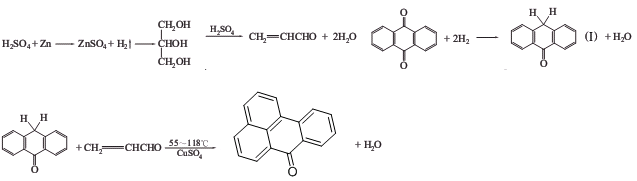
Preparation of Benzanthrone: it is prepared by reaction of anthracenequinone with glycerol. Firstly, anthracenequinone is reducted with iron to generate hydroxy-anthrone. Meanwhile, glycerol reacts with concentrated sulfuric acid to get acrolein. Secondly, hydroxy anthraquinone is mixed with acrolein with the presence of sulfuric acid to form new ring. At last, product is obtained by oxidation with sulfuric acid.
Uses of Benzanthrone: it is used to produce 3-nitro-benz[de]anthracen-7-one. The reaction needs reagent 88% nitric acid and solvent nitrobenzene at the temperature of 50 °C for 3 hours. The yield is about 74.6%.
![Benzanthrone is used to produce 3-nitro-benz[de]anthracen-7-one.](/UserFilesUpload/Uses of Benzanthrone.png)
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C2C(=C1)C3=CC=CC4=C3C(=CC=C4)C2=O
2. InChI: InChI=1S/C17H10O/c18-17-14-8-2-1-7-12(14)13-9-3-5-11-6-4-10-15(17)16(11)13/h1-10H
3. InChIKey: HUKPVYBUJRAUAG-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 290mg/kg (290mg/kg) | Russian Pharmacology and Toxicology Vol. 40, Pg. 137, 1977. | |
| rat | LD50 | intraperitoneal | 1500mg/kg (1500mg/kg) | Russian Pharmacology and Toxicology Vol. 40, Pg. 137, 1977. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View