-
Name
Benzo[e]pyrene
- EINECS 205-892-7
- CAS No. 192-97-2
- Article Data43
- CAS DataBase
- Density 1.286g/cm3
- Solubility 0.984ug/L(25 oC)
-
Melting Point
177-180 °C(lit.)
- Formula C20H12
- Boiling Point 467.5°C at 760 mmHg
- Molecular Weight 252.315
- Flash Point 228.6°C
- Transport Information
- Appearance
-
Safety
Questionable carcinogen with experimental tumorigenic data. Experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Analytical Methods:
For occupational chemical analysis use NIOSH: Polynuclear Aromatic Hydrocarbons (HPLC), 5506; (GC), 5515.
- Risk Codes 45-50/53-67-65-38-11
- Molecular Structure
- Hazard Symbols T,N,Xn,F
- Synonyms 1,2-Benzopyrene;1,2-Benzpyrene;4,5-Benzopyrene;4,5-Benzpyrene;NSC 89273;
- PSA 0.00000
- LogP 5.73720
Synthetic route
-
-
68151-09-7
9-Hydroxy-1,2,3,6,7,8,9,10,11,12-decahydrobenzopyrene

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield palladium on activated charcoal at 300 - 320℃; for 2h; 86% -
-
86439-17-0
cis-8b,9,10,11,12,12a-hexahydrobenzopyrene

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 6h; Heating; 78% With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 6h; Heating; 56% -
-
121617-90-1
exo-4,5-dihydrobenzopyreno-2',3':4,5-norbornane

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield In gas under 0.01 - 0.05 Torr; sublimation at 150-200 degC, 4 h to a quartz tube of 800 degC; 69% -
-
149296-39-9
spiro

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 6h; Heating; 60% -
-
77320-76-4
trans-4,5-Dihydroxy-4,5-divinyl-4,5-dihydropyrene

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With trichlorophosphate In pyridine for 0.166667h; Heating; 52% -
-
157729-37-8
3-(4H-cyclopentaphenanthrylidene)-1,5-bis(trimethylsilyl)-1,4-pentadiyne

-
A
-
129-00-0
pyrene

-
B
-
192-97-2
benzo[e]pyrene

-
C
-
203-64-5
4H-Cyclopenta[def]phenanthrene

-
D
-
27208-37-3
cyclopenta[c,d]pyrene

-
E
-
203-12-3
benzo[ghi]fluoranthene

-
F
-
5821-51-2
Coarannulen
Conditions
Conditions Yield With hydrogen In gas at 900℃; Product distribution; electrically heated vertical laboratory tubular furnace; A n/a
B n/a
C n/a
D n/a
E n/a
F 15%...Expand
-
-
157729-37-8
3-(4H-cyclopentaphenanthrylidene)-1,5-bis(trimethylsilyl)-1,4-pentadiyne

-
A
-
129-00-0
pyrene

-
B
-
192-97-2
benzo[e]pyrene

-
C
-
27208-37-3
cyclopenta[c,d]pyrene

-
D
-
5821-51-2
Coarannulen
Conditions
Conditions Yield With hydrogen In gas at 900℃; electrically heated vertical laboratory tubular furnace; Further byproducts given; A n/a
B n/a
C n/a
D 15%...Expand
-
-
68151-08-6
9-Oxo-1,2,3,6,7,8,9,10,11,12-decahydrobenzopyrene

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With ethanol; sodium Erhitzen des Reaktionsprodukts mit Selen auf 320-340grad; -
-
857580-09-7
6-propyl-benz[de]anthracen-7-one

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With zinc Conditions
Conditions Yield With hydrogen fluoride at 3 - 20℃; Destillation des Reaktionsprodukts in Gegenwart von Quecksilber; With hydrogen fluoride at 3 - 20℃; Destillation des Reaktionsprodukts in Gegenwart von Quecksilber; Conditions
Conditions Yield With sodium methylate Multistep reaction; -
-
114468-92-7
4-phenyl-2,3-phenantrenedicarboxylic acid anhydride

-
A
-
192-97-2
benzo[e]pyrene

-
B
-
4325-78-4
4-phenylphenanthrene
Conditions
Conditions Yield With silica gel at 950℃; for 1.4h; Mechanism; A 84 % Chromat.
B 12 % Chromat.Conditions
Conditions Yield With quinoline; sodium methylate 1.) RT, 3 h, 2.) 180 deg C, 16 h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; ...Expand

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With air Oxidation; Formation of xenobiotics; 
-
A
-
193-39-5
Indeno[1,2,3-cd]pyrene

-
B
-
192-97-2
benzo[e]pyrene

-
C
-
198-55-0
PERYLENE

-
D
-
191-24-2
Benzo[ghi]perylene
Conditions
Conditions Yield With air at 600 - 900℃; Oxidation; Formation of xenobiotics; Further byproducts given; ...Expand

-
A
-
205-99-2
benzo[e]acephenanthrylene

-
B
-
207-08-9
Benzo[k]fluoranthene

-
C
-
218-01-9
chrysene

-
D
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With air Oxidation; Formation of xenobiotics; Further byproducts given; ...Expand Conditions
Conditions
Conditions Yield at 5 - 20℃; Erhitzen des Reaktionsprodukts mit Quecksilber; at 5 - 20℃; Erhitzen des Reaktionsprodukts mit Quecksilber; ...Expand Conditions
Conditions
Conditions Yield With sodium chloride; zinc(II) chloride at 300℃; Conditions
Conditions Yield With soda lime; copper Erhitzen unter vermindertem Druck; -
-
64-17-5
ethanol

-
-
68151-08-6
9-Oxo-1,2,3,6,7,8,9,10,11,12-decahydrobenzopyrene

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield Erhitzen des Reaktionsprodukts mit Selen auf 320-340grad; ...Expand

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
A
-
192-97-2
benzo[e]pyrene

-
B
-
198-55-0
PERYLENE
Conditions
Conditions Yield at 300℃; ...Expand

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield Decomposition; Formation of xenobiotics; pyrolysis; Conditions
Conditions Yield With air Oxidation; Formation of xenobiotics; Further byproducts given; ...Expand Conditions
Conditions
Conditions Yield With air at 800℃; Oxidation; Formation of xenobiotics; Further byproducts given. Title compound not separated from byproducts; ...Expand Conditions
Conditions
Conditions Yield With oxygen at 1023.85 - 1161.85℃; Formation of xenobiotics; Further byproducts given. Title compound not separated from byproducts; ...Expand

-
A
-
129-00-0
pyrene

-
B
-
205-99-2
benzo[e]acephenanthrylene

-
C
-
206-44-0
fluoranthene

-
D
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With oxygen at 1036.85 - 1162.85℃; Formation of xenobiotics; Further byproducts given. Title compound not separated from byproducts; ...Expand
-
-
115-10-6
Dimethyl ether

-
A
-
129-00-0
pyrene

-
B
-
193-39-5
Indeno[1,2,3-cd]pyrene

-
C
-
192-97-2
benzo[e]pyrene

-
D
-
203-64-5
4H-Cyclopenta[def]phenanthrene
Conditions
Conditions Yield With air at 840℃; under 19501.6 Torr; Formation of xenobiotics; high pressure combustion; Further byproducts given. Title compound not separated from byproducts; ...Expand Conditions
Conditions
Conditions Yield With air at 880℃; under 18001.4 Torr; Formation of xenobiotics; high pressure combustion; Further byproducts given. Title compound not separated from byproducts; ...Expand
-
-
192-97-2
benzo[e]pyrene

-
-
26105-52-2
3-Bromobenzopyrene
Conditions
Conditions Yield With benzyltrimethylazanium tribroman-2-uide; zinc(II) chloride In chloroform at 20℃; for 3h; 96% With benzyltrimethylazanium tribroman-2-uide; zinc(II) chloride In chloroform at 20℃; for 3h; 96% -
-
192-97-2
benzo[e]pyrene

-
-
66788-08-7
benzopyrene-4,5-dione
Conditions
Conditions Yield With tert.-butylhydroperoxide; Ru(2,4,13,15-tetraphenyl-1,5,12,16-tetraaza-tricyclo[14.2.2.06,11]eicosa-4,6(11),7,9,12-pentaene)Cl2 In acetonitrile for 6h; Reagent/catalyst; Irradiation; 80% With sodium periodate; rhodium(III) chloride hydrate In dichloromethane; water; acetonitrile at 30 - 40℃; 38% Multi-step reaction with 2 steps
1: (i) OsO4, Py, (ii) aq. NaHSO3, Py
2: DDQ
View Scheme-
-
192-97-2
benzo[e]pyrene

-
-
77508-03-3
3,6-Dibromobenzopyrene
Conditions
Conditions Yield With bromine In chlorobenzene for 0.25h; Ambient temperature; 78% With bromine In chlorobenzene -
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
1039768-31-4
[Ru2(η6-p-cymene)2(C6H2O4)Cl2]

-
-
192-97-2
benzo[e]pyrene

-
-
2923-28-6
silver trifluoromethanesulfonate
Conditions
Conditions Yield In methanol byproducts: AgCl; a mixt. of complex and Ag-salt in methanol was stirred at room temp. for2 h, filtered, triazine-compound and aromatic molecule were added, the mixt. was stirred at room temp. for 24 h; the solvent was removed under vac., the residue was taken up in CH2Cl2, filtered, concd., diethyl ether was added; elem. anal.; 68% ...Expand
-
-
14104-20-2
silver tetrafluoroborate

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield In dichloromethane; acetone byproducts: AgCl; reaction under N2: addn. of AgBF4 to a soln. of Ru-complex in acetone, stirring for 15 min at room temp., filtn. to remove AgCl, evapn. of solvent under vac., addn. of CH2Cl2 along with the ligand, refluxing for 48 h; filtn., washing with CH2Cl2 and Et2O, elem. anal.; 56% ...Expand Conditions
Conditions
Conditions Yield With aluminium trichloride In benzene -
-
192-97-2
benzo[e]pyrene

-
-
95430-01-6
3,5,8,10-Tetrachlo-1,2-benzpyren
Conditions
Conditions Yield With chlorine -
-
192-97-2
benzo[e]pyrene

-
-
92387-50-3
1,2,3,6,7,8,9,10,11,12-decahydrobenzopyrene
Conditions
Conditions Yield With sodium In pentan-1-ol -
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With o-tetrachloroquinone; hydrogen bromide Conditions
Conditions Yield With aluminium trichloride In dichloromethane -
-
192-97-2
benzo[e]pyrene

-
-
24909-10-2
cis-4,5-Dihydroxy-4,5-dihydrobenzopyrene
Conditions
Conditions Yield (i) OsO4, Py, (ii) aq. NaHSO3, Py; Multistep reaction; With osmium(VIII) oxide In pyridine; benzene for 120h; in the dark; Conditions
Conditions Yield With bromine In chlorobenzene for 0.5h; Product distribution; Ambient temperature; various amounts of bromine and times of the reaction; With bromine In chlorobenzene for 0.5h; Ambient temperature; Yield given. Yields of byproduct given; Conditions
Conditions Yield With nitric acid at 0℃; for 90h; Yield given. Yields of byproduct given; -
-
192-97-2
benzo[e]pyrene
Conditions
Conditions Yield With N-fluoro-2,4-dinitroimidazole In 1,2-dichloro-ethane for 72h; Heating; Yield given; Yields of byproduct given. Title compound not separated from byproducts; ...Expand
Benzo(e)pyrene Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 32 (1983),p. 225.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 3 (1973),p. 137.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program.
Benzo(e)pyrene Analytical Methods
For occupational chemical analysis use NIOSH: Polynuclear Aromatic Hydrocarbons (HPLC), 5506; (GC), 5515.
Benzo(e)pyrene Specification
The IUPAC name of this chemical is Benzo(e)pyrene, and it has its cas register number 192-97-2. This is a kind of colorless crystals or white crystalline solid and is insoluble in water. Besides, its product categories are including a-banalytical standards; alpha sort; aromaticsalphabetic; ba - bhchemical class; hydrocarbons; neatsanalytical standards; pahsenvironmental standards.
The characteristics of this chemical are as following: (1)XLogP3: 6.4; (2)Exact Mass: 252.0939; (3)MonoIsotopic Mass: 252.0939; (4)Topological Polar Surface Area: 0; (5)Heavy Atom Count: 20; (6)Formal Charge: 0; (7)Complexity: 336; (8)Covalently-Bonded Unit Count: 1.
Use of Benzo(e)pyrene: Benzo(e)pyrene could react to produce 3,6-Dibromobenzo[e]pyrene, in the following condtion: reagent: Br2; solvent: chlorobenzene; reaction time: 15 mins; field: 78%; other condition: Ambient temperature.
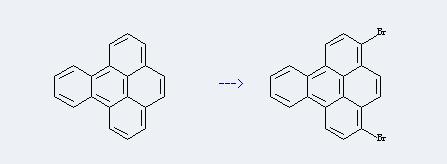
Producing method of Benzo(e)pyrene: 9-Hydroxy-1,2,3,6,7,8,9,10,11,12-decahydrobenzo[e]pyrene could react to produce Benzo(e)pyrene, in the following condtion: catalytic agent: 10percent Pd/C; reaction time: 2 hours; reaction temp.: 300 - 320 ℃; field: 86%.
.jpg)
When you are dealing with this chemical, you should be very cautious. For being a kind of toxic chemical, it could at low levels cause damage to health, and may cause damage to health, and could even cause cance. Besides, it is dangerous for the environment, for it may present an immediate or delayed danger to one or more components of the environment, and may cause long-term adverse effects in the aquatic environment. What's more, it is highly flammable, for it may catch fire in contact with air, only needing brief contact with an ignition source, and it has a very low flash point or evolve highly flammable gases in contact with water. Therefore, you should take the following instructions. Avoid exposure - obtain special instructions before using and avoid releasing to the environment and refer to special instructions/safety data sheet at the same time. Besides, if in case of accident or if you feel unwell, seek medical advice immediately (show the label where possible); If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label; And this material and its container must be disposed of as hazardous waste.
Additionally, you could obtain the molecular structure through converting the following datas:
(1)Canonical SMILES: C1=CC=C2C(=C1)C3=CC=CC4=C3C5=C(C=CC=C25)C=C4
(2)InChI: InChI=1S/C20H12/c1-2-8-16-15(7-1)17-9-3-5-13-11-12-14-6-4-10-18(16)20
(14)19(13)17/h1-12H
(3)InChIKey: TXVHTIQJNYSSKO-UHFFFAOYSA-NRelated Products
- Benzo(e)pyrene
- 1929-73-3
- 1929-82-4
- 1929-89-1
- 192997-48-1
- 193001-91-1
- 193002-24-3
- 193002-25-4
- 193003-92-8
- 193003-99-5
- 19301-39-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View