-
Name
difluoromethanediyl dihypofluorite
- EINECS
- CAS No. 16282-67-0
- Article Data3
- CAS DataBase
- Density 1.507g/cm3
- Solubility
- Melting Point
- Formula CF4 O2
- Boiling Point 16.5°Cat760mmHg
- Molecular Weight 120.003
- Flash Point °C
- Transport Information
- Appearance
- Safety A strong oxidant. Potentially hazardous reactions with organic or easily oxidized materials. Potentially explosive reactions with trans-dichloroethylene, tetrafluoroethylene and possibly other haloalkanes. When heated to decomposition it emits toxic fumes of F−.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Hypofluorousacid, difluoromethylene ester (9CI); Methanediol, difluoro-, dihypofluorite(8CI); Bis(fluorooxy)difluoromethane; Bis(fluorooxy)perfluoromethane;Difluoromethylene bis(hypofluorite); Difluoromethylene hypofluorite
- PSA 18.46000
- LogP 1.33890
Bis(fluoroxy)perfluoromethane Chemical Properties
Molecule structure of Bis(fluoroxy)perfluoromethane (CAS NO.16282-67-0):
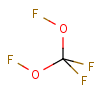
IUPAC Name of Bis(fluoroxy)perfluoromethane (CAS NO.16282-67-0): [Difluoro(fluorooxy)methyl] hypofluorite
Molecular Weight: 120.003113 g/mol
Molecular Formula: CF4O2
Density: 1.507 g/cm3
Boiling Point: 16.5 °C at 760 mmHg
Index of Refraction: 1.212
Molar Refractivity: 10.79 cm3
Molar Volume: 79.6 cm3
Polarizability: 4.27×10-24 cm3
Surface Tension: 10.8 dyne/cm
Enthalpy of Vaporization: 25.28 kJ/mol
Vapour Pressure: 1030 mmHg at 25 °C
XLogP3-AA: 1.9
H-Bond Acceptor: 6
Exact Mass: 119.983442
MonoIsotopic Mass: 119.983442
Topological Polar Surface Area: 18.5
Heavy Atom Count: 7
Complexity: 47.7
Canonical SMILES: C(OF)(OF)(F)F
InChI: InChI=1S/CF4O2/c2-1(3,6-4)7-5
InChIKey of Bis(fluoroxy)perfluoromethane (CAS NO.16282-67-0): GMLJCMXFMUEABC-UHFFFAOYSA-N
Bis(fluoroxy)perfluoromethane Safety Profile
A strong oxidant. Potentially hazardous reactions with organic or easily oxidized materials. Potentially explosive reactions with trans-dichloroethylene, tetrafluoroethylene and possibly other haloalkanes. When heated to decomposition it emits toxic fumes of F−.
Related Products
- Bis(fluoroxy)perfluoromethane
- 16283-36-6
- 16284-60-9
- 162848-16-0
- 162848-22-8
- 162848-23-9
- 162849-95-8
- 162853-41-0
- 162856-35-1
- 16285-74-8
- 162881-26-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View