-
Name
Bismuth subcarbonate
- EINECS 227-567-9
- CAS No. 5892-10-4
- Density 6.86 g/mL at 25 °C(lit.)
- Solubility Practically insoluble in water, alcohol. Soluble in nitric acid, hydrochloric acid, concentrated acetic acid.Soluble in mineral acid and glacial acetic acid. Insoluble in water.
- Melting Point 308(分解)oC
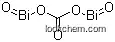
- Formula CBi2O5
- Boiling Point 333.6oC at 760mmHg
- Molecular Weight 509.97
- Flash Point 169.8oC
- Transport Information
- Appearance white fine powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Bismuth subcarbonate (TN);Bismuth oxycarbonate;2,4-Dioxa-1,5-dibismapentane,1,3,5-trioxo-;carbonic acid; oxobismuth;Basic bismuth carbonate;Bismuth subcarbonate (JAN/USP);Dibismuth carbonate dioxide;1,3,5-Trioxo-2,4-dioxa-1,5-dibismapentane;
- PSA 69.67000
- LogP -0.16940
Bismuth subcarbonate Specification
The Bismuth subcarbonate, with the CAS registry number 5892-10-4, is also known as 1,3,5-Trioxo-2,4-dioxa-1,5-dibismapentane. It belongs to the product category of Inorganics. Its EINECS number is 227-567-9. This chemical's molecular formula is CBi2O5 and molecular weight is 509.97. What's more, its systematic name is bis(oxobismuthanyl) carbonate. Its classification code is Protectant [topical]. It should be sealed and stored in a cool and dry place. Moreover, it should be protected from light and moisture. It is used in pharmaceutical industry, and it can also be used as an analytical reagent or to prepare bismuth salts. You should not breathe dust. When using it, you must avoid contact with eyes.
Physical properties of Bismuth subcarbonate are: (1)#H bond acceptors: 5; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 4; (4)Polar Surface Area: 69.67 Å2.
Preparation: this chemical can be prepared by nitric acid bismuth as raw materials, which then has a metathesis reaction with sodium carbonate solution. Then after washing, centrifugal separation, drying, smashing, the end product Bismuth subcarbonate is got.
6 Bi(NO3)3 + 4 Na2CO3 + H2O → (BiO)2CO3·1/2H2O + Na2CO3 + 4 CO2
You can still convert the following datas into molecular structure:
(1)SMILES: C(=O)(O[Bi]=O)O[Bi]=O
(2)Std. InChI: InChI=1S/CH2O3.2Bi.2O/c2-1(3)4;;;;/h(H2,2,3,4);;;;/q;2*+1;;/p-2
(3)Std. InChIKey: MGLUJXPJRXTKJM-UHFFFAOYSA-L
Related Products
- Bismuth
- Bismuth 2-ethylhexanoate
- Bismuth Acetate
- Bismuth arsphenamine sulfonate
- Bismuth bromide
- Bismuth citrate
- Bismuth dimethyl dithiocarbamate
- Bismuth hydroxide
- Bismuth nitrate
- Bismuth nitrate oxide
- 5892-11-5
- 589-21-9
- 5892-21-7
- 58924-00-8
- 58928-39-5
- 589-29-7
- 5893-05-0
- 58-93-5
- 589-35-5
- 5893-66-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View